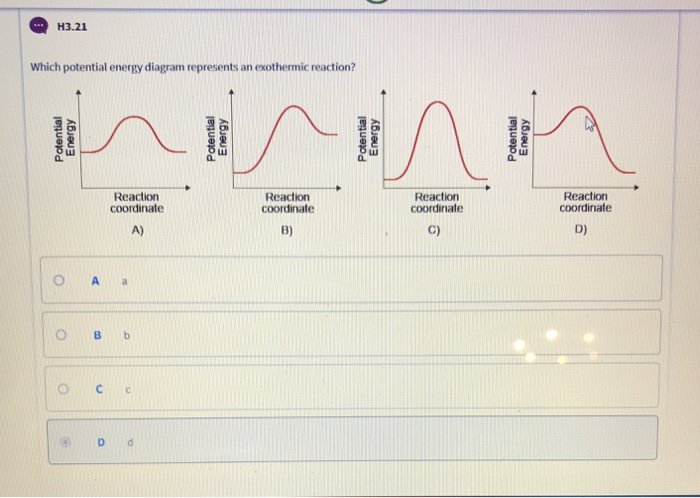
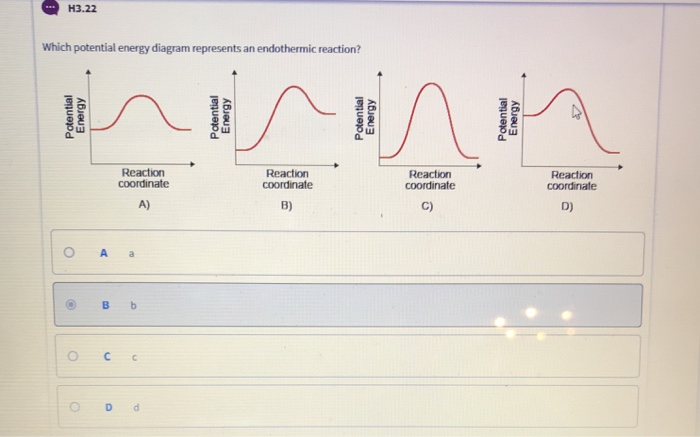
35 for an exothermic reaction, what would the potential energy diagram most likely look like?
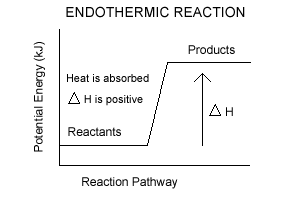
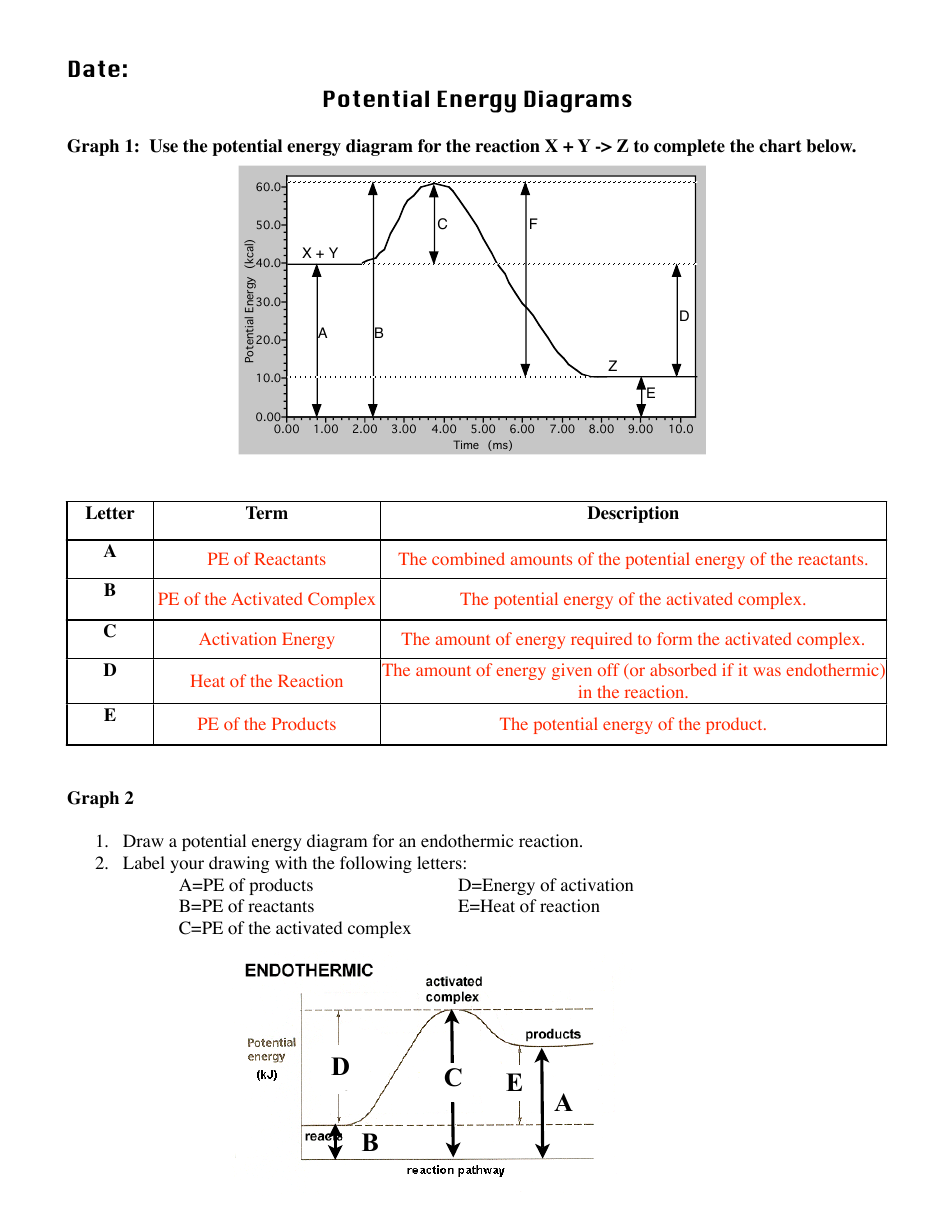
endothermic : A process or reaction which absorbs energy. enthalpy: The enthalpy change is the amount of heat released or absorbed when a chemical reaction occurs at constant pressure. exothermic: A process or reaction which releases energy. heat: Energy transferred between two systems as a result of a temperature difference.
± Cell Potential and Free Energy. Calculate the standard free-energy change at 25 ∘C for the following reaction: Mg(s)+Fe2+(aq)→Mg2+(aq)+Fe(s) Delta G = -nFEo. Calculate the standard cell potential at 25 ∘C for the reaction X(s)+2Y+(aq)→X2+(aq)+2Y(s) E∘ = 3.42 V n= 2 mol. Use the table of standard reduction potentials given above to calculate the equilibrium constant at …
Natural gas is a fossil energy source that formed deep beneath the earth's surface. Natural gas contains many different compounds. The largest component of natural gas is methane, a compound with one carbon atom and four hydrogen atoms (CH4). Natural gas also contains smaller amounts of natural gas liquids (NGL, which are also hydrocarbon gas ...

For an exothermic reaction, what would the potential energy diagram most likely look like?
I would like to know if it is not strange to have this address in accounthash, because I wonder if the person does not want to take expenses with that, I am learning to deconstruct a contract so if...
02.05.2014 · The rate of an exothermic reaction is decreased by decreasing the temperature. A refrigeration system was provided to keep the MIC at about 30°F. Had the tank been operated at that temperature, the reaction rate would have been much lower and the event may have been far less catastrophic. Ironically, the refrigeration system was turned off months before the …
Plant cell ribosome definition. This is the organelle responsible for protein synthesis of the cell. Its found in the cell cytoplasm in large numbers and a few of them called functional ribosomes can be found in the nucleus, mitochondria, and the cell chloroplast. Its made up of ribosomal DNA (rDNA) and cell proteins.
For an exothermic reaction, what would the potential energy diagram most likely look like?.
Students explore the physics exploited by engineers in designing today's roller coasters, including potential and kinetic energy, friction and gravity. First, they learn that all true roller coasters are completely driven by the force of gravity and that the conversion between potential and kinetic energy is essential to all roller coasters. Second, they consider the role of friction in ...
In the energy charging and discharging processes, the metal oxide CaAl 0.2 Mn 0.8 O 2.9-δ (CAM28) undergoes a reversible, high temperature redox cycle including an endothermic oxygen-releasing reaction and exothermic oxygen-incorporation reaction. Concentrated solar radiation heats the redox-active oxide particles under partial vacuum to drive ...
Carbon monoxide (chemical formula CO) is a colorless, odorless, tasteless, flammable gas that is slightly less dense than air.Carbon monoxide consists of one carbon atom and one oxygen atom. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl.It is a key ingredient in many processes in industrial chemistry.
Among them some most outstanding and important factors need to listed are current economic situation, laws, surrounding infrastructure, and customer demands. Economic situation Economy is one of the most determining factors to the success of the company even though it is an external element.
Cellular respiration is the chemical reaction in which glucose and oxygen are turned into water, carbon dioxide, and energy (ATP). In this reaction, glucose and oxygen are reactants, while water ...
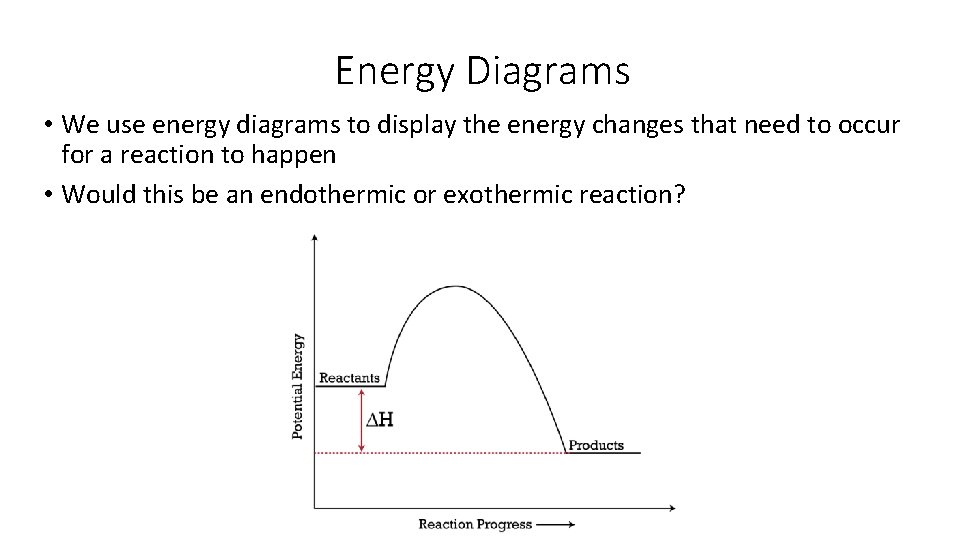
This physical reaction appears to be irreversible, and occurs because the ball has lower potential energy at the bottom of the hill than it does at the top. The gap in the potential energy is related to the “extent” and spontaneity of this reaction. As we have observed before, nature tends to go to a lower energy state. By analogy, we will consider the driving force for a …
UPDATE: Liberty Scott, bless 'im, outlines what a National Party political platform that meant something might look like; a realistic nine-point plan from Luxon, Willis et al that, first of all, would avoid backsliding on the National Policy Statement (NPS) on the Medium Density Residential Standards (MDRS), and then begins with:
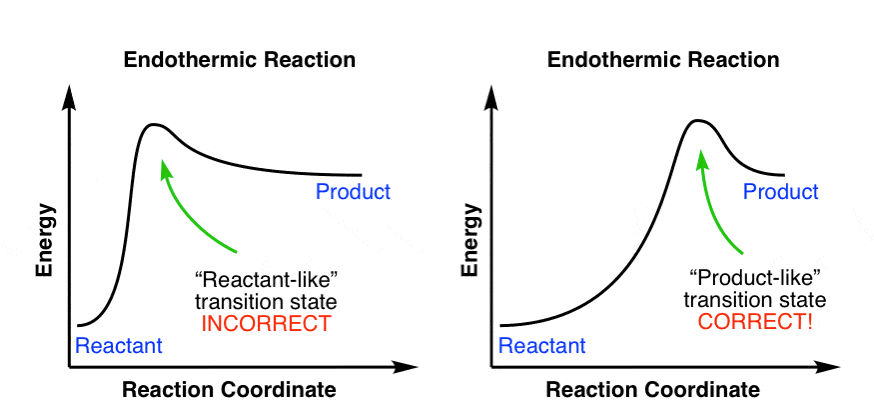
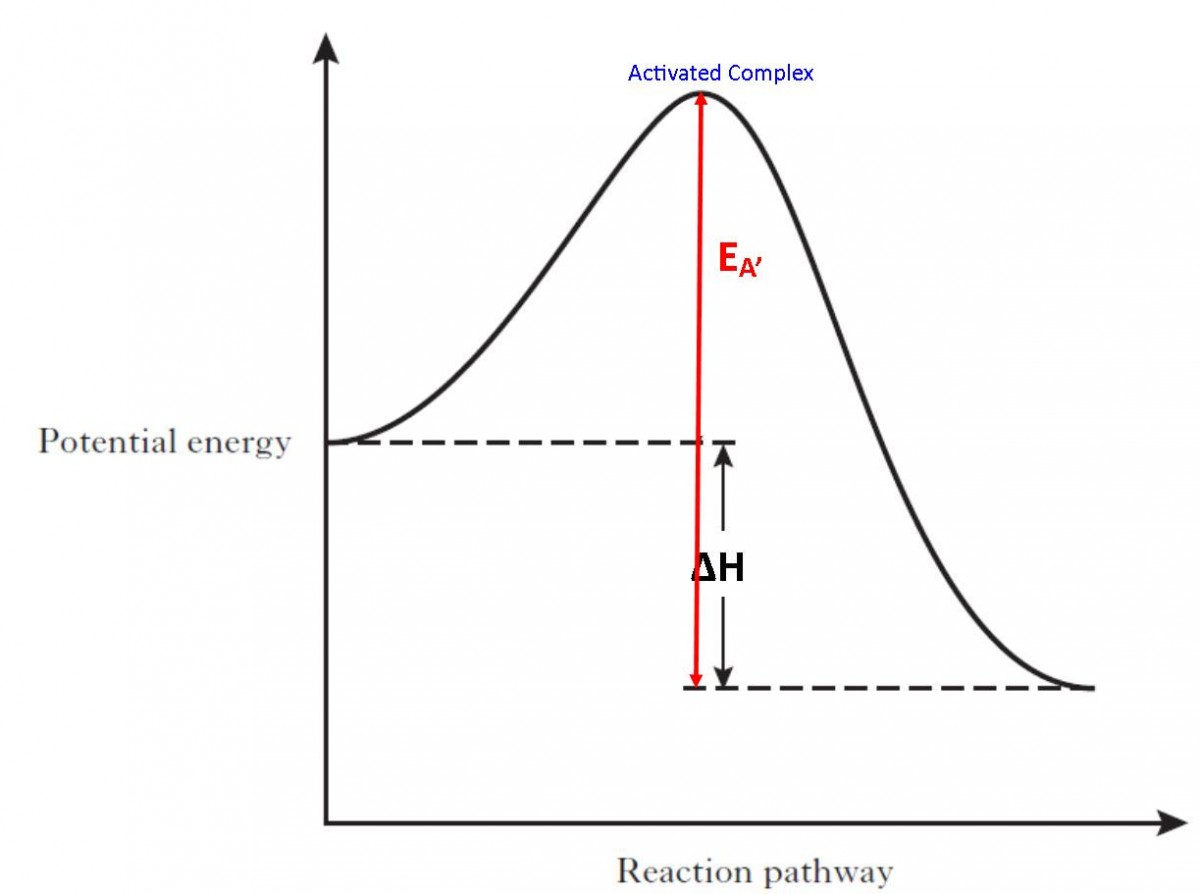
The horizontal axis in our potential energy diagram is labelled as the reaction coordinate; how is this opaque term best described? E. It conveys the movement from a positioning of the atoms in the reactant configuration to a positioning of the atoms in the product configuration.
Betaine derivatives, especially esters, are compounds of interest for the development of a more sustainable fine chemistry, as they are widely available from biomass and currently produced as side-products from various industries (among which, sugar production). In this publication, we studied the impact of carbon chain length on three considered reaction mechanisms for the esterification of ...
The students at Vienna Elementary sold donuts every day at school for 6 months. The table below shows the earnings for the first 6 weeks. If the pattern continues, how much will th... SAT, 24.12.2021 05:40. A special 8-sided die is marked with the numbers 1 to 8. It is rolled 15 times with the results shown in the table.... SAT, 24.12.2021 05:40.
mar 17 2021 middot determine your reaction rsquo s products and reactants any chemical reaction involves two categories of chemicals
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities.Some of this chemical energy is stored in carbohydrate molecules, such as sugars and starches, which are synthesized from carbon dioxide and water - hence the name photosynthesis, from the Greek ...
Potential Energy is energy of position (technically position in a gradient "field"). The most familiar form of potential energy is gravitational, such as Newton's apple as it hung in the tree. More relevant to chemistry is the potential energy due to position in an electric or magnetic field, such as solvated ions, or atoms transferring charge when forming compounds or molecules. …
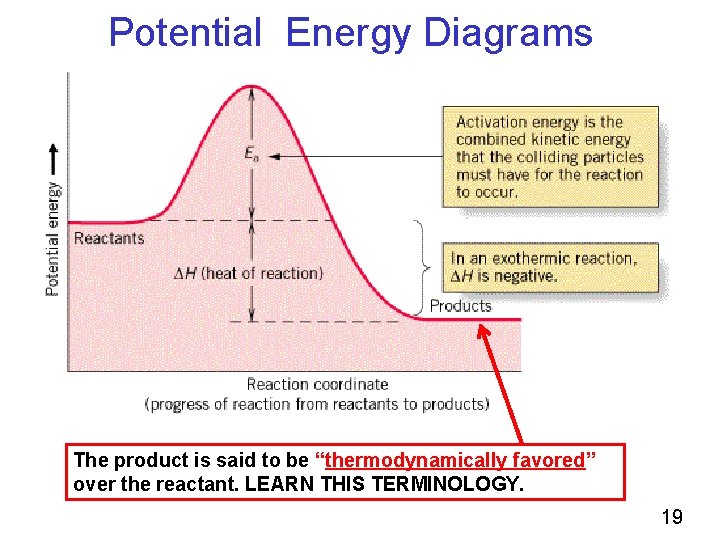
The amount of energy amount of heat evolved or absorbed in a reaction carried out a… enthalpy change when equation quantities of materials react un…. The enthalpy change of a reaction is roughly equivalent to the amount of energy lost or gained during the reaction. You'll see at the end that you arrive back at. Enthalpy of Reaction.
Chapter 17 reaction rates worksheet answers
The meaning of negative result on reaction energy is that the reaction is exothermic. Our analysis shows that the N-N bond cleavage has an exothermic reaction. Our previous result on Ni embedded on graphene with a single vacancy is that forward activation barriers are 0.85 eV and 0.51 eV for the N-H bond and the N-N bond scissions, and the ...
To unravel these types of questions, it will help if you know the different types of analogies that the test-makers might use: Opposites Analogies (e.g., fire and ice, tired and energetic, crying and laughing, etc.).; Object and Classification Analogies (e.g., red and color, knife and kitchenware, truck and vehicle, etc.).; Object and Related Object Analogies (e.g., dog and puppy, kangaroo and ...
The Gibbs free energy G is the thermodynamic potential that tells us which way a reaction goes at a given set of physical conditions--neither the enthalpy change nor the entropy change for a reaction alone can provide us with this information. The two measures of energy (enthalpy H and entropic energy TS) are brought together in the Gibbs free energy equation: (the chemical …
Generic potential energy diagram showing the effect of a catalyst in a hypothetical exothermic chemical reaction X + Y to give Z. The presence of the catalyst opens a different reaction pathway (shown in red) with a lower activation energy. The final result and the overall thermodynamics are the same. Catalysts work by providing an (alternative) mechanism …
a run-away chemical reaction (chemical energy), the release of compressed gas or steam (pressure; high temperature), entanglement of hair or clothing in rotating equipment (kinetic energy), or. contact with electrodes of a battery or capacitor (electrical energy). Please see the OSH Answers on Hazard Identification for more information.
Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. Performance Performance. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user ...
Potential Energy Diagrams for Chemical Reactions ... Sketch out an activation energy diagram for a multistep mechanism involving a rate-determining step, and relate this to the activation energy of the overall reaction. Write the rate law expression for a two-step mechanism in which the rate constants have significantly different magnitudes. Write the rate law expression for a …
21.02.2016 · Exothermic reactions require special consideration due to their potential to runaway (temperature rises from heat of reaction being released, increasing reaction rate, releasing more heat, and so on). The reactor must be designed such that temperature can be precisely controlled and the reaction shut down if temperature control is lost. The use of …





















0 Response to "35 for an exothermic reaction, what would the potential energy diagram most likely look like?"
Post a Comment