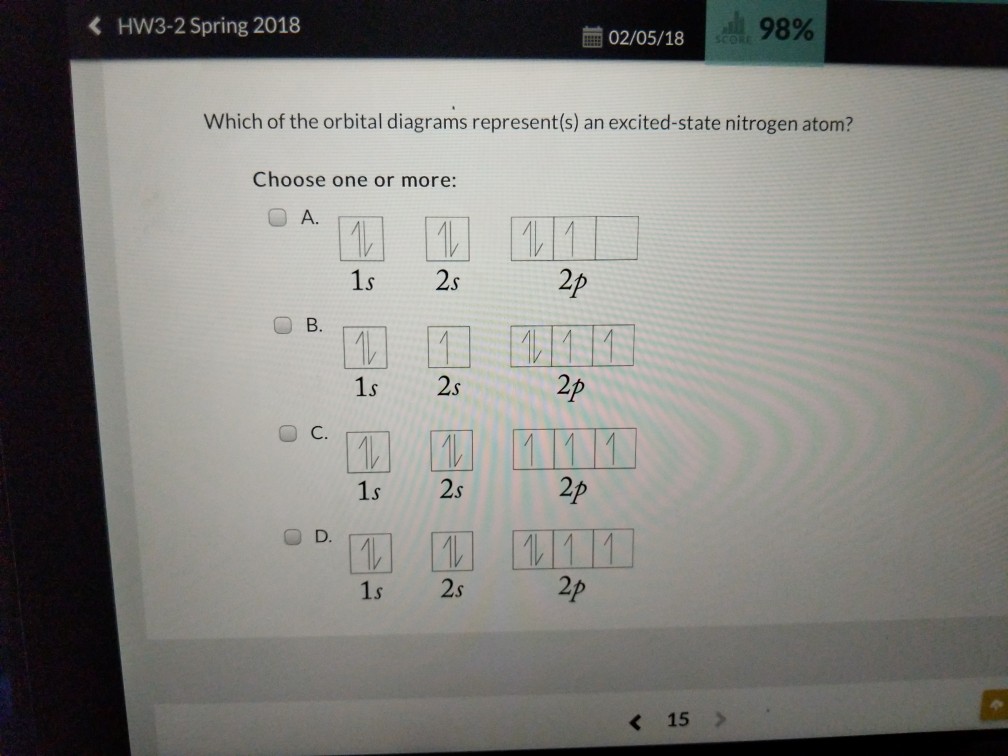
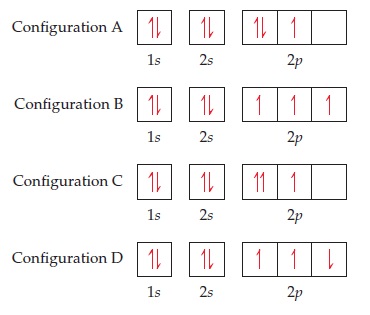
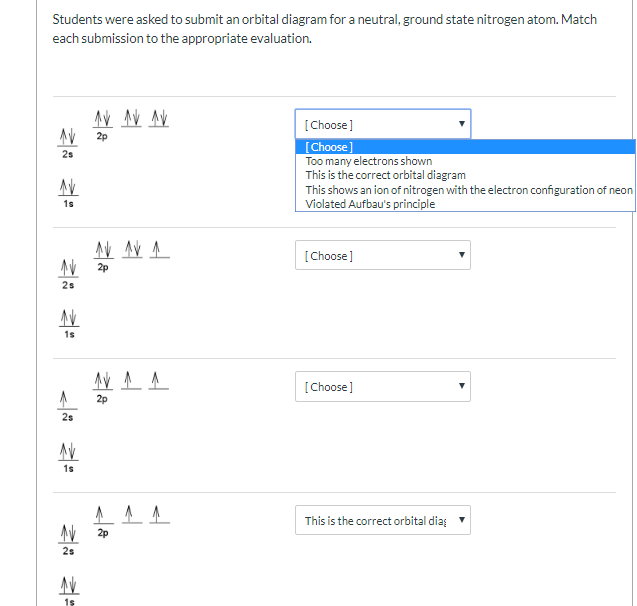
35 the orbital diagram for a ground state nitrogen atom is
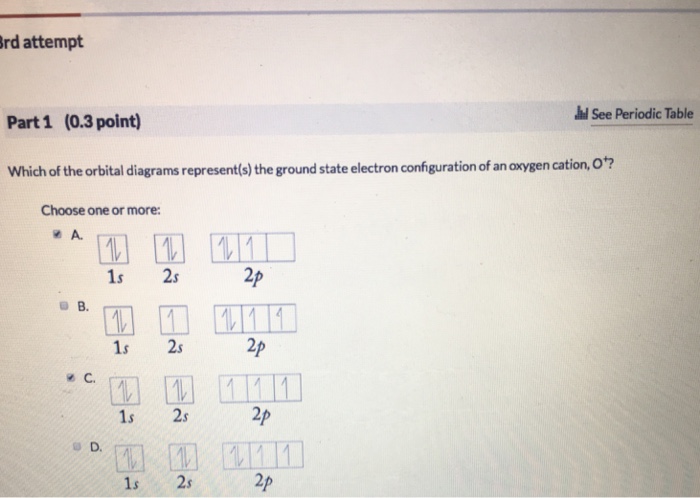
The orbital diagram for a ground-state nitrogen atom is. 1s (up down) 2s (up down) 2p (up, up, up) Electrons in an orbital with l = 3 are in a/an. ... For all atoms of the same element, the 2s orbital is larger than the 1s orbital. true. An FM radio station broadcasts at a frequency of 101.7 MHz. Calculate the wavelength Chemistry questions and answers. The orbital diagram for a ground-state... The orbital diagram for a ground-state oxygen atom is в. 1, т. 11- С.Т 1Тут — D. 1 1 1 1 1 E. Тут, ТІ ТІ ооооо.
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbital s and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
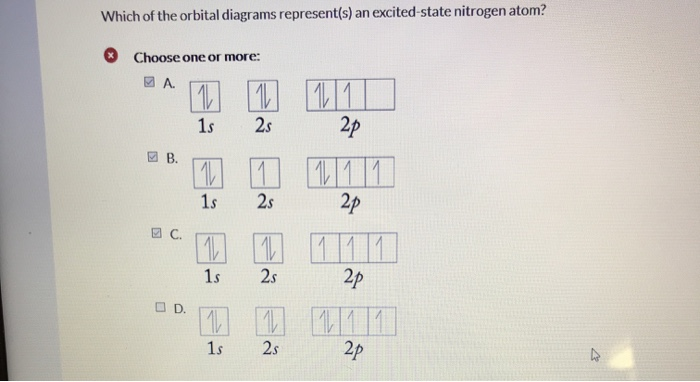
The orbital diagram for a ground state nitrogen atom is
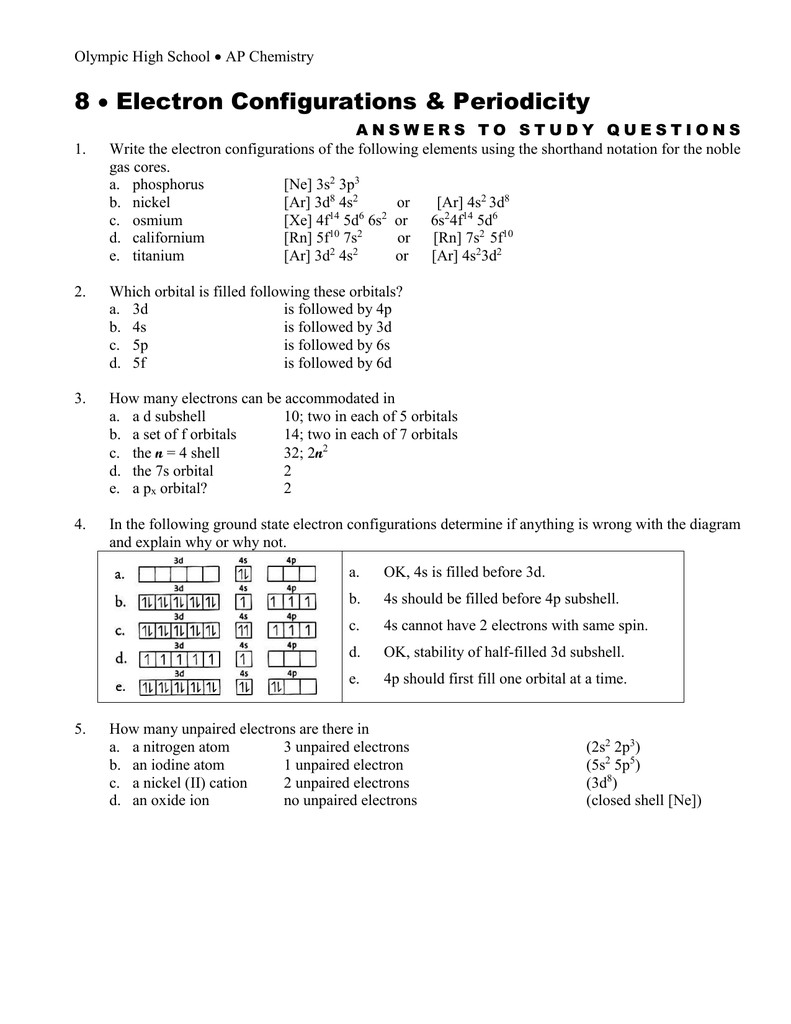
The ground state electron configuration of p is ne3s23p3. Electrons in an orbital with l 3 are in a a. Answer to the orbital diagram for a ground state nitrogen atom is. Example k 1s 2 2s 2 2p 6 4s 1 or k. C has two unpaired electrons in its ground state. This subshell is full. Create the atomic orbital diagram for nitrogen. Master chem 1311 ... Which electron orbital diagram is written correctly for an atom without any violations? ^ ^ ^^^ Which is the correct electron configuration for a nitrogen atom? 1s22s22p3. Which is the correct electron configuration for an oxide ion? ... What is the principal quantum number for the outermost electrons in a Te atom in the ground state? 5. What orbital diagram correctly represents the outermost principal energy level of a nitrogen atom in the ground state? 1S22S23P3 1S22S23P3 What atom is represented in the following orbital diagram ...
The orbital diagram for a ground state nitrogen atom is. The orbital diagram for a ground state nitrogen atom is. C has two unpaired electrons in its ground state. A possible set of quantum numbers for the last electron added to complete an atom of germanium in its ground state is a. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. The orbital diagram for a ground-state nitrogen atom is. 1s 2s 2p ↑↓ ↑↓ ↑ ↑ ↑ The orbital diagram for a ground state carbon atom is. 1s 2s 2p ↑↓ ↑↓ ↑ ↑ How many unpaired electrons does a ground-state atom of sulfur have? 2. The electron configuration of a ground-state Co atom is ... 1s2,2s2,2p6,3s2,3d9 [Ar]4s2,3d7 [Ne]3s2,3d7 [Ar]4s1,3d5 ... The orbital diagram for a ground-state nitrogen atom is 1s 2s 2p A ↿⇂ ↿⇂ ↿ . ↿ . ↿ B ↿⇂ ↿ . ↿⇂ ↿ . C ↿⇂ ↿⇂ ↿ . ↿ . ↿ The orbital diagram for a ground state nitrogen is a a b b c c d d the electron configuration. It depends on the atom. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. The ground state electron configuration of p is ne3s23p3. Nitrogen is the seventh element with a total of 7 electrons.
The orbital diagram for a ground state nitrogen is a a b b c c d d the electron configuration of an atom shows a the number of isotopes possible. C has two unpaired electrons in its ground state. The following orbital filling diagram represents an excited state rather than the ground state of an atom. It depends on the atom. 40 draw the orbital diagram for the ion co2+. Written By Kathy W. Blatt. Wednesday, November 17, 2021 Add Comment Edit. Answer (1 of 3): The atomic orbital s of oxygen are uni for mly lower in energy than the corresponding atomic orbital s of element C because of the increased stability of the electrons in oxygen. A student draws the orbital diagram below for the 3d electrons in a V atom. What, if anything, is incorrect about the drawing? {^v}{^ }{ }{ }{ } 3d a. It violates the aufbau principle b. it violates the pauli exclusion principle c. it violates the heisenburg uncertainty principle d. it violates hunds rule e. there is nothing wrong about the picture Orbital Filling Diagram s. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbital s are shown as circles (or squares) and orbital s within a sublevel are drawn next to each o the r horizontally.
The orbital diagram for a ground-state nitrogen atom is. Ans: A. Category: Medium Section: 7.8. 41. The orbital diagram for a ground-state oxygen atom is. Ans: D. Category: Medium Section: 7.8. 42. The orbital diagram for a ground state carbon atom is. Ans: D. Category: Medium Section: 7.8. 43. Which ground-state atom has an electron ... Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... orbital diagram for sodium confirms that the 3s sublevel is lower in energy than the 3p sublevel. The s sublevel is located lower on the page than the p sublevel. 10. The lowest potential energy arrangement of electrons in an atom is called the ground state. Ground state electron configurations can be predicted by a strict set of rules known as the
The orbital diagram for a ground state nitrogen atom is 1s 2s 2p A B C D A A B from CHEM 101 at Qatar University
The orbital diagram for a ground-state nitrogen atom is | 1s 25 A.TV C. | | 치서 cm 0 e o000 치어 리 리 리 기 기tmuo | 소 ; Question: The orbital diagram for a ground-state nitrogen atom is | 1s 25 A.TV C. | | 치서 cm 0 e o000 치어 리 리 리 기 기tmuo | 소
energy level diagram for the ground state of a nitrogen atom In each case. Energy level diagram for the ground state of a. School Panther Creek High; Course Title CHEMISTRY PHYSICAL C; Uploaded By EarlBoulderWildcat7. Pages 8 This preview shows page 6 - 8 out of 8 pages.
The orbital diagram for a ground state nitrogen atom. Write the electron configuration for the following elements. The ground state electron configuration of p is ne3s23p3. The remaining three electrons will go in the 2p orbital. You may use the full electron shell notation or the inner shell noble gas method.
Ground or Excited State for Nitrogen. In question 1.69 (b), there is a picture which shows the electron configuration for Nitrogen. There are two arrows for the 1s orbital, 2 arrows in the 2s orbital, and one arrow in each of the three 2p orbitals. The question asks us to determine whether the electron configuration represents the excited state ...
h. ground state. A particle of electromagnetic radiation with no mass that carries a quantum of energy. ... Write the orbital diagram and complete the electron configuration for each atom: nitrogen. Orbital: Nitrogen - 7 Configuration: 1s2, 2s2, 2p3 ...
This problem has been solved! What is the orbital diagram for the valence electrons in a ground state atom of nitrogen? Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Ans :- The orbital dia ….
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Here is the full molecular orbital diagram for N 2.
What orbital diagram correctly represents the outermost principal energy level of a nitrogen atom in the ground state? 1S22S23P3 1S22S23P3 What atom is represented in the following orbital diagram ...
Which electron orbital diagram is written correctly for an atom without any violations? ^ ^ ^^^ Which is the correct electron configuration for a nitrogen atom? 1s22s22p3. Which is the correct electron configuration for an oxide ion? ... What is the principal quantum number for the outermost electrons in a Te atom in the ground state? 5.
The ground state electron configuration of p is ne3s23p3. Electrons in an orbital with l 3 are in a a. Answer to the orbital diagram for a ground state nitrogen atom is. Example k 1s 2 2s 2 2p 6 4s 1 or k. C has two unpaired electrons in its ground state. This subshell is full. Create the atomic orbital diagram for nitrogen. Master chem 1311 ...

At the Johnson Space Center during the memorial for the Columbia astronauts, President George W. Bush states, “Each of these astronauts had the daring and discipline required of their calling. Each of them knew that great endeavors are inseparable from great risks. And each of them accepted those risks willingly, even joyfully, in the cause of discovery.”





















0 Response to "35 the orbital diagram for a ground state nitrogen atom is"
Post a Comment