38 orbital filling diagram for nitrogen
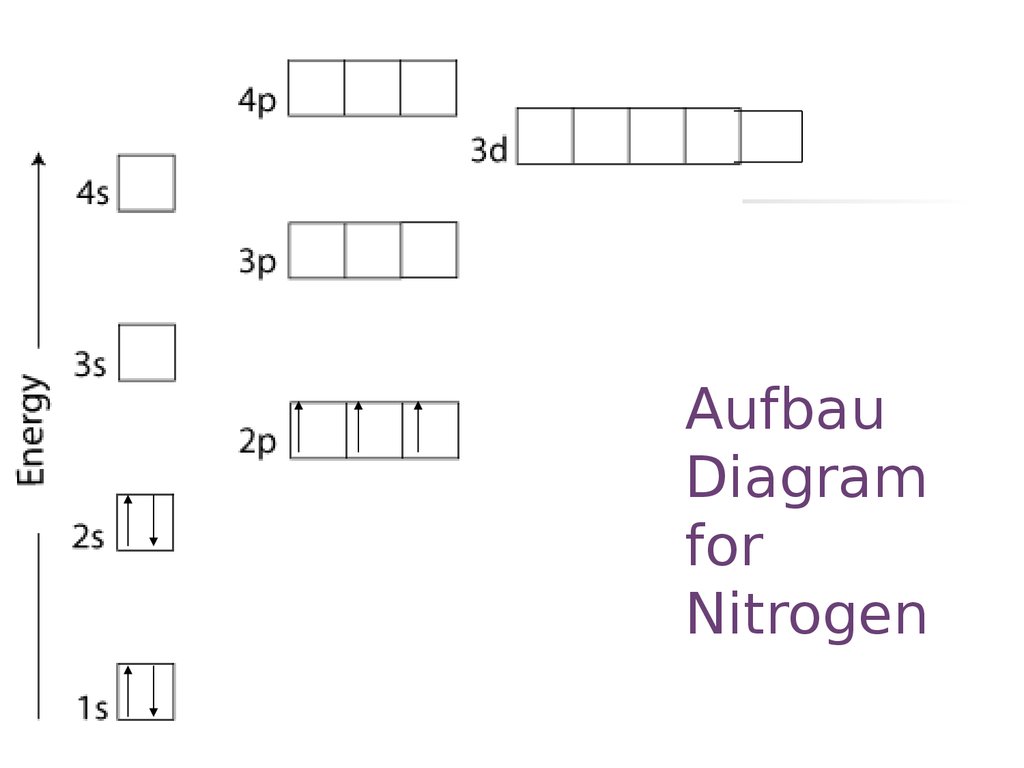
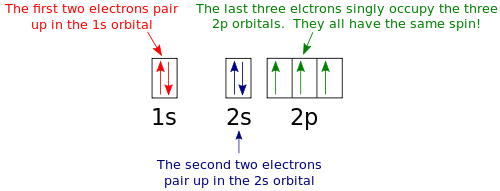
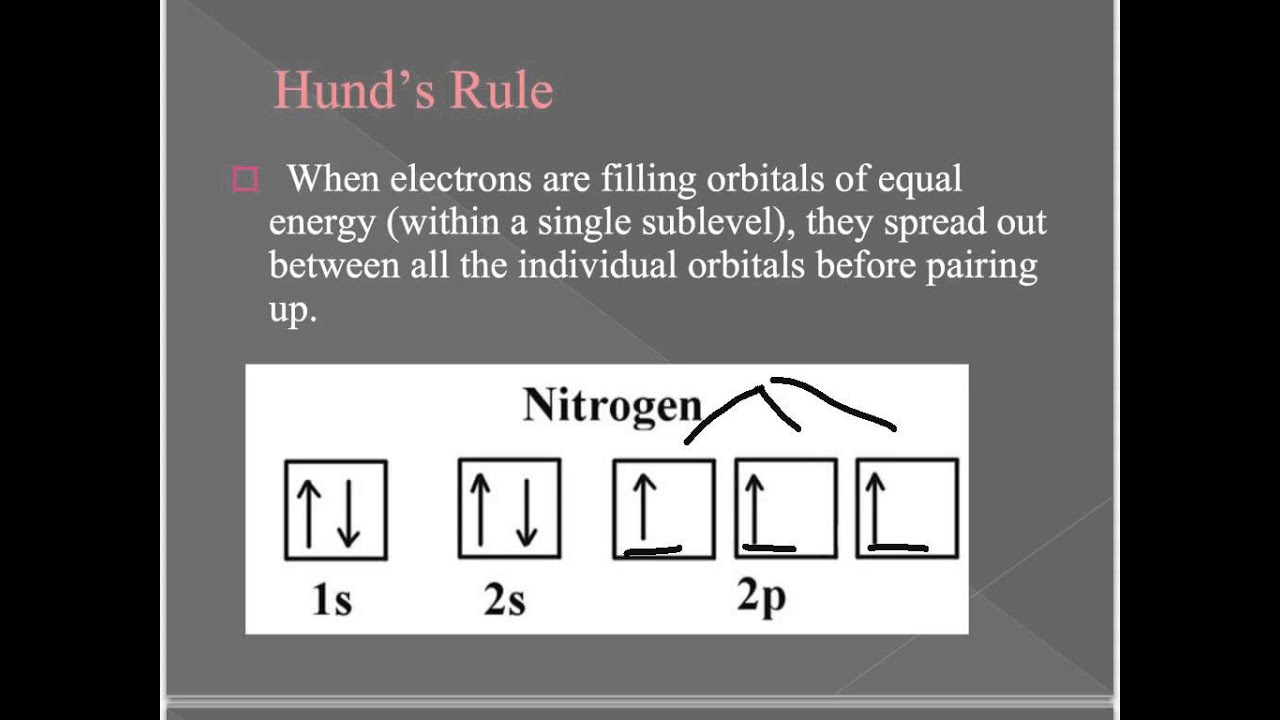
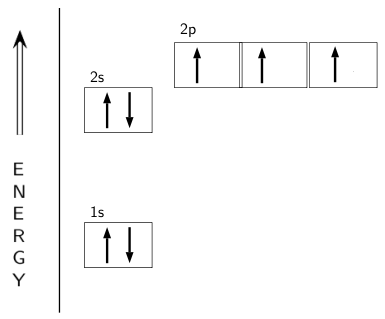
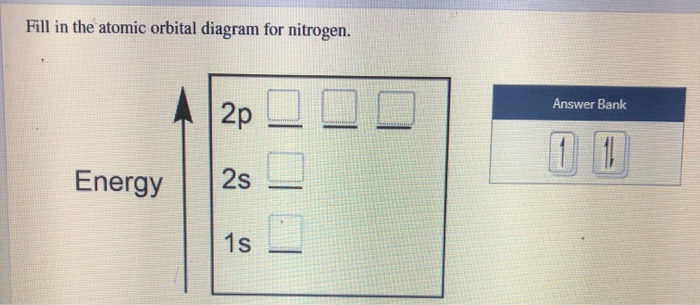
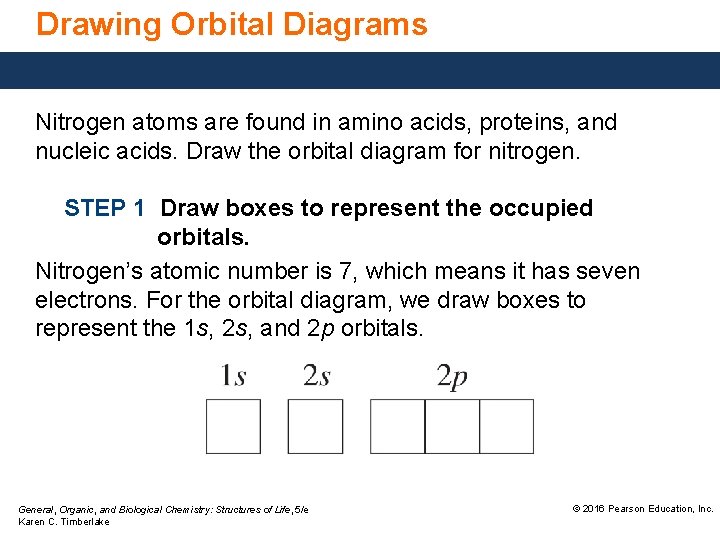
Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
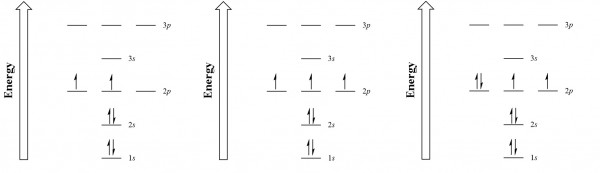
Carbon (atomic number 6) has six electrons. Four of them fill the 1s and 2s orbitals. The remaining two electrons occupy the 2p subshell. We now have a choice of filling one of the 2p orbitals and pairing the electrons or of leaving the electrons unpaired in two different, but degenerate, p orbitals. The orbitals are filled as described by Hund's rule: every degenerate orbital is first ...

Orbital filling diagram for nitrogen
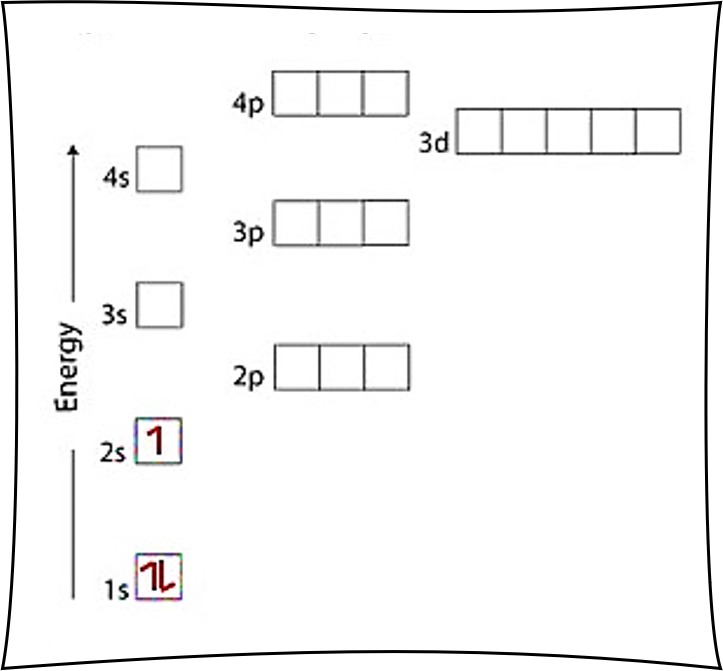
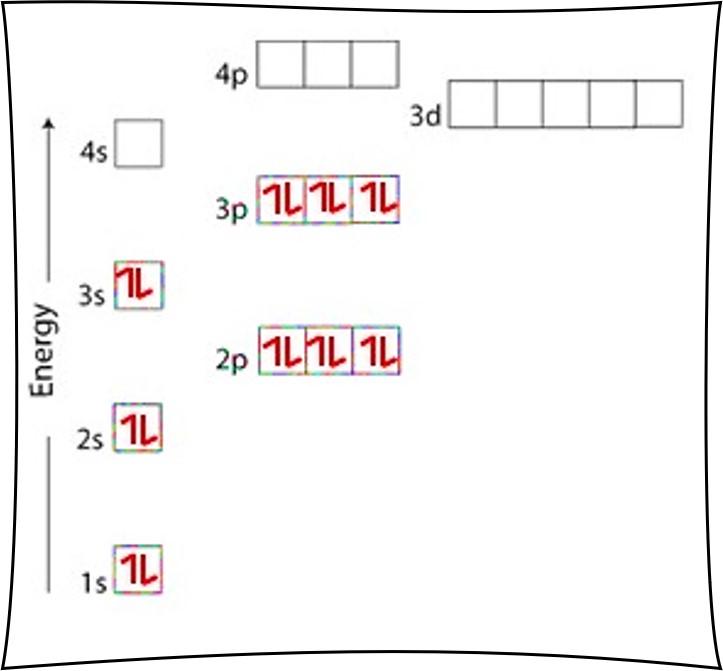
Nitrogen electron configuration is 1s 2 2s 2 2p 3.The period of nitrogen is 2 and nitrogen is a p-block element. The electron configuration of nitrogen(N) and the orbital diagram is the main topic of this article. The periodic table shows us that nitrogen (N) has an atomic number of 7. As a result, a neutral nitrogen atom will have 7 electrons. In orbital filling diagrams, s-sublevels have 1 orbital and p ... Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. 1s²2s²2p³
Orbital filling diagram for nitrogen. To see this video, other videos, chemistry education text, and practice problems visit my website. Website is 100% FREE to use.http://scientifictutor.org/ Orbital Filling Diagram for Nitrogen. show the orbital filling diagram for rm n nitrogen best answer the electronic configuration for nitrogen atom is 1s 2 2s 2 2p 3 lowest energy state will have two electrons in s shell which is spherical in shape one spin up and another spin down chemistry problem please help show the orbital filling diagram for nitrogen stack the subshells inorder of energy ... Chemistry questions and answers. Part A Use the orbital-filling diagram to show the electron configuration of helium, He. Use the buttons at the top of the tool to add sublevels. Click within an orbital to add electrons. View Available Hint (s) 15 23 22 35 3p 3d 4s 4p 4d 4 55 5P 5d 5f 6s 6p 6d 75 7P 7d Part B Use the orbital-filling diagram to ... Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s.
Draw an orbital diagram for scandium Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for S Status: Resolved. home / study / science / chemistry / chemistry questions and answers ... The orbital filling diagram for carbon Again, we start with the electron configuration, which is 1s²2s²2p². As we've seen, this means that there are 2 electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbitals. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for S (sulfur). Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
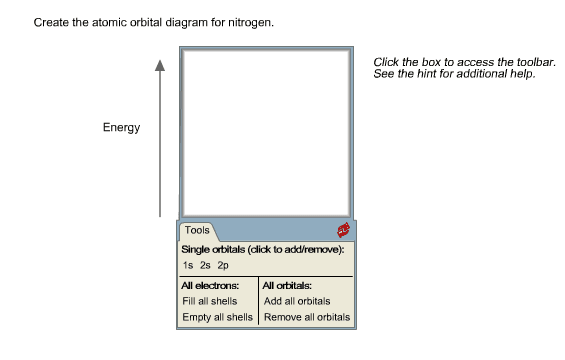
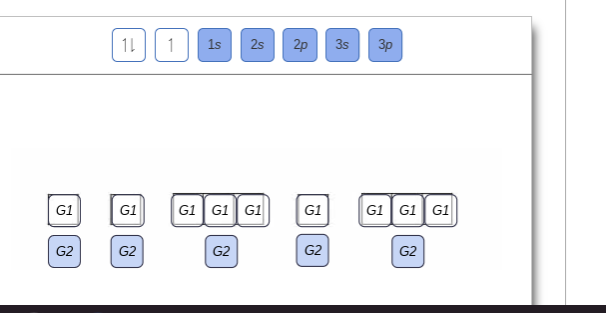
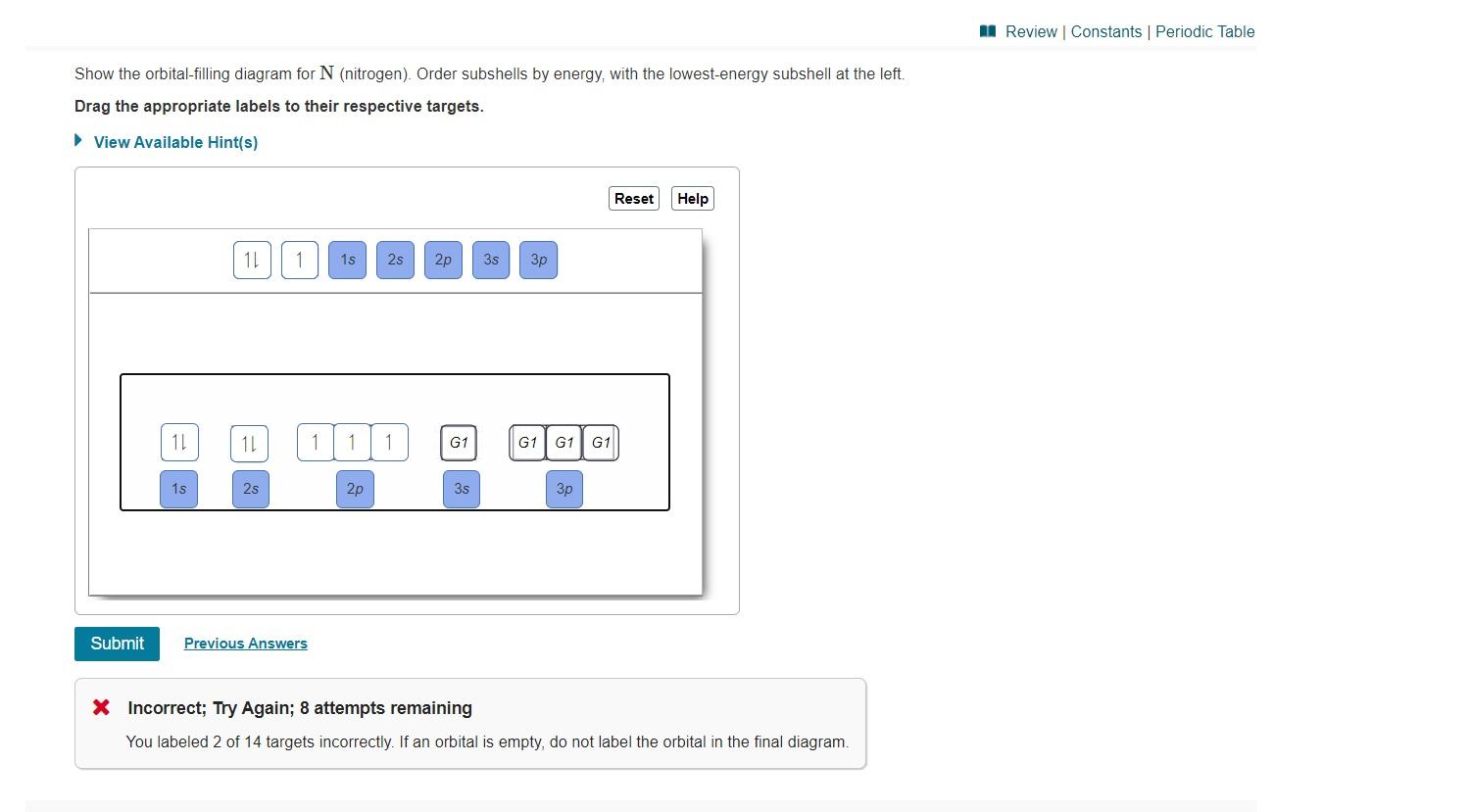
Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na) Ground or Excited State for Nitrogen. In question 1.69 (b), there is a picture which shows the electron configuration for Nitrogen. There are two arrows for the 1s orbital, 2 arrows in the 2s orbital, and one arrow in each of the three 2p orbitals. The question asks us to determine whether the electron configuration represents the excited state ... Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 11 || 1 18 2s 2p 3s ap Group 1 GT G1 G1 G1 GI GT G161 G2 G2 G2 G2 G2 ; Question: Show the orbital-filling diagram for N (nitrogen ... Show the orbital filling diagram for n nitrogen for n nitrogen. Show more show less. Show the orbital filling diagram for s sulfur. Electron configurations list the orbitals from lower to higher energy. Show the orbital filling diagram for s sulfur. Website is 100 free to use. Click within the orbital to add electrons. 1 3 5 7.
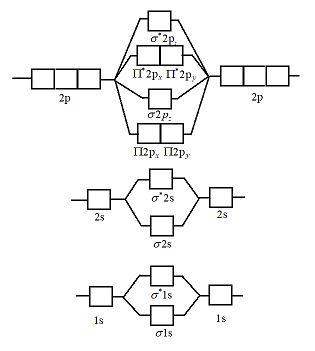
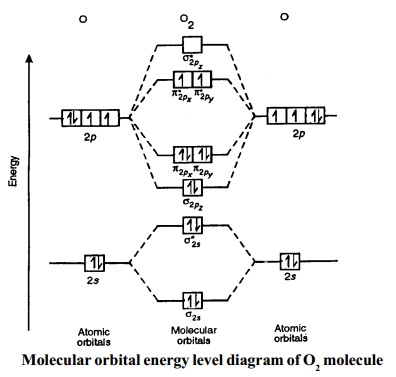
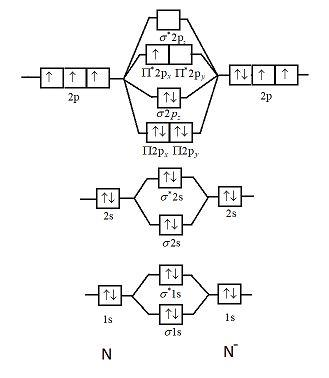
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Here is the full molecular orbital diagram for N 2.
Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top . Show the orbital-filling diagram for \rm Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy.
Chemistry Q&A Library Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 1L 1 1s 2s 2p 3s Зр G1 G1 G1 G1 G1 G1 G1 G1 G1 G2 G2 G2 G2 G2 Part C Show the orbital-filling diagram for S (sulfur).
If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two ...
Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p . Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be.Given the same amount of absorbed solar energy coming in, the amount of IR escaping to space at the top of the ...
Show the orbital-filling diagram for (bromine).Status: Resolved. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top%(15). 1. Describe the two differences between a 2p x orbital and a 3p y orbital.

Difference Between Orbital Diagram And Electron Configuration Compare The Difference Between Similar Terms
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
Molecular orbital energy level diagrams -Hydrogen, Hypothetical, Nitrogen, Oxygen. The filling of molecular orbitals is governed by the following principles. (i)Aufbau principle (ii)Pauli's exclusion principle and (iii)Hund's rule of maximum multiplicity. Now, let us consider some examples of homo nuclear diatomic molecules.
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. 1s²2s²2p³
The periodic table shows us that nitrogen (N) has an atomic number of 7. As a result, a neutral nitrogen atom will have 7 electrons. In orbital filling diagrams, s-sublevels have 1 orbital and p ...
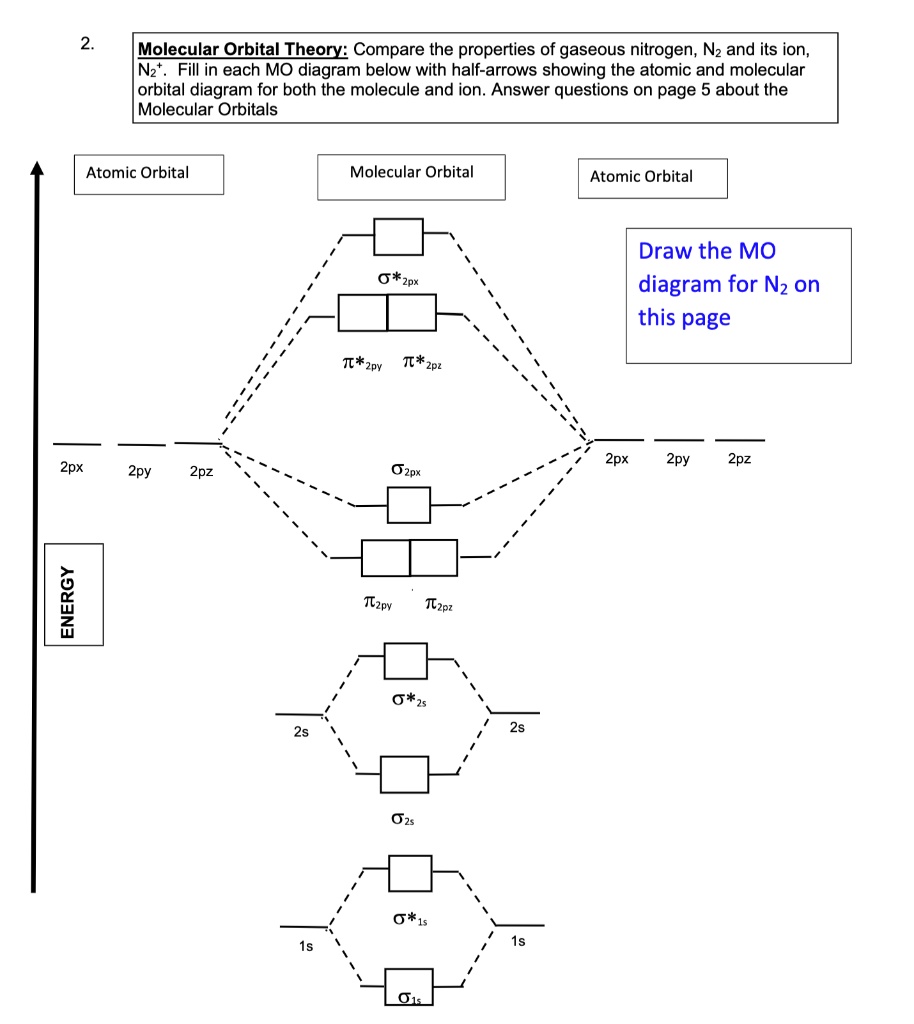
Solved Molecular Qrbital Theory Compare The Properties Of Gaseous Nitrogen Nz And Its Ion Nz Fill In Each Mo Diagram Below With Half Arrows Showing The Atomic And Molecular Orbital Diagram For Both The
Nitrogen electron configuration is 1s 2 2s 2 2p 3.The period of nitrogen is 2 and nitrogen is a p-block element. The electron configuration of nitrogen(N) and the orbital diagram is the main topic of this article.
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes

Review I Constants I Show The Orbital Filling Diagram For S Sulfur Order Subshells By Energy With Homeworklib

Difference Between Orbital Diagram And Electron Configuration Compare The Difference Between Similar Terms








0 Response to "38 orbital filling diagram for nitrogen"
Post a Comment