36 solid liquid phase diagram
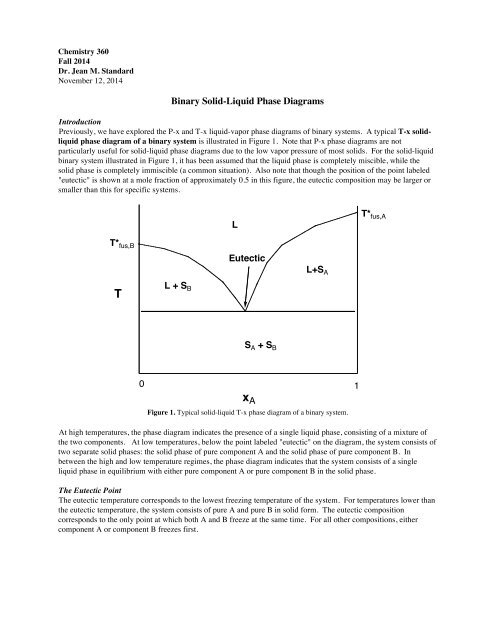
PDF Liquid-Solid Phase Diagrams - Texas A&M University Liquid-Solid Phase Diagrams Solid and liquid phases can be present below the boiling point (e.g., immiscible pair of metals right up to their melting points (As and Bi) 2-component liquid at temperature a1: (1) a1 6 a2 System enters "Liquid+B" pure solid B comes out of solution, remaining liquid richer in A (2) a2 6 a3 More solid B forms, equal Binary Solid-Liquid Phase Diagram - ENG 5304 - Foundations ... In this lab, a solid-liquid phase diagram was constructed using several cooling curves of a. mixture at different compositions. The arrest and breaks of the cooling curves were used to. determine the temperatures for the points at each composition. The phase diagram was used to. determine the eutectic point of the mixture.
PDF EXPERIMENT 4 - Constructing a Solid-Liquid Phase Diagram ... The objective is to determine the liquid-solid phase diagram and associated thermodynamic parameters of a two component (binary) compound system. To accomplish this, you must rst run calibration runs with materials that have know melting points and heats of fusion. Because of time constraints, this data will be available on the class website.

Solid liquid phase diagram
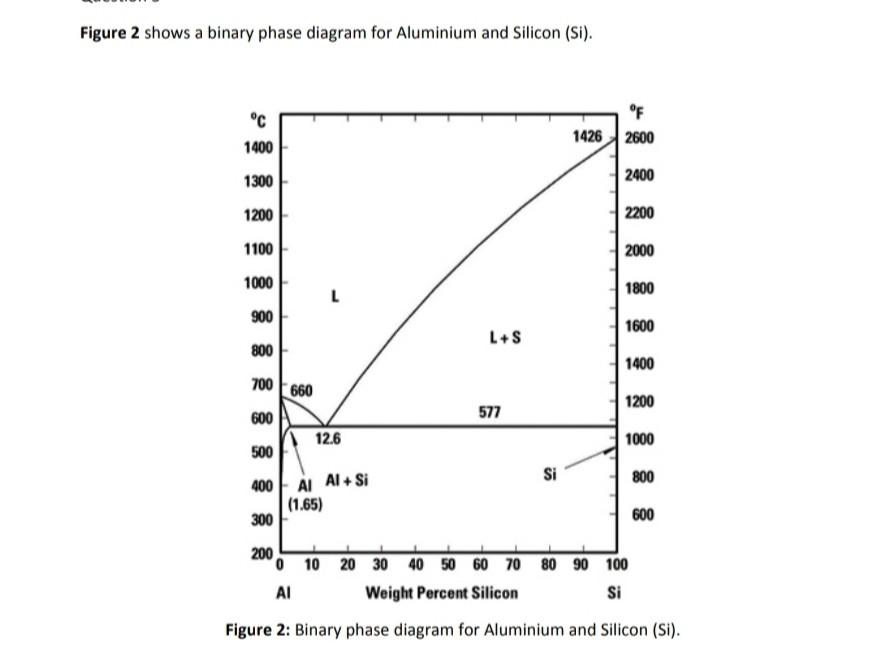
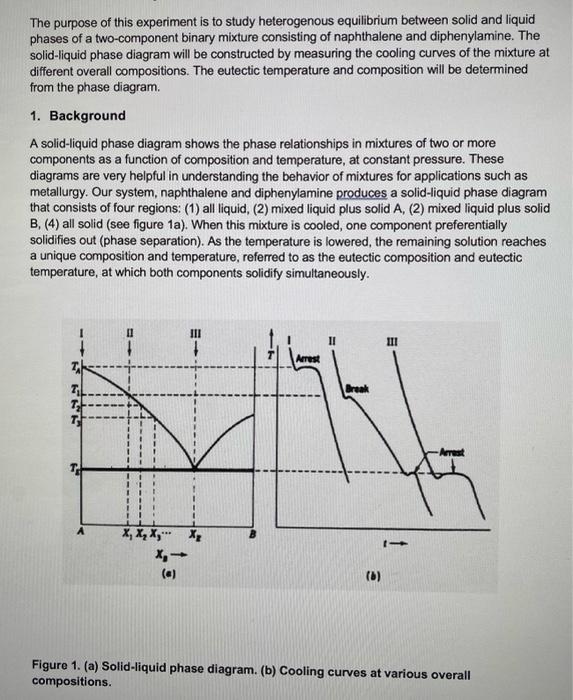
PDF Experiment 1 Solid - Liquid Phase Diagram The binary solid-liquid phase diagram for the naphthalene-diphenylamine system will be constructed from cooling curves. Several mixtures of different ratios of the two components will be melted, and temperature versus time curves will be plotted as the mixtures cool. PDF Simple Solid - Liquid Phase Equilibria An understanding of solid liquid phase equilibria is required to describe and calculate phase diagrams of interest in both fundamental materila science and applications such as crystallizer design. A very simple phase diagram is shown below. For this system the two solid phase do not mix (form intercallated crystals) or form solid compounds. PDF Phase Diagrams, Solid Solutions, Phase Transformations Phase Diagrams: composition of phases At TA= 1320°C: Only Liquid (L) present CL= C0 ( = 35 wt% Ni) At TB= 1250°C: Both and L present At TD= 1190°C: Only Solid ( ) present C = C0( = 35 wt% Ni) C L = C liquidus ( = 32 wt% Ni) C = C solidus ( = 43 wt% Ni) 18 • Rule 3:If we know T and Co, then we know: --the amount of each phase (given in wt%).
Solid liquid phase diagram. Solid/liquid phase diagram of the ammonium sulfate ... Solid/liquid phase diagram of the ammonium sulfate/glutaric acid/water system. Beyer KD(1), Pearson CS, Henningfield DS. Author information: (1)Department of Chemistry, University of Wisconsin-La Crosse, La Crosse, Wisconsin 54601, USA. kbeyer@uwlax.edu We have studied the low temperature phase diagram and water activities of the ammonium ... Solid-liquid Phase Diagrams: Salt Solution SOLID-LIQUID PHASE DIAGRAMS: SALT SOLUTION This page looks at the phase diagram for mixtures of salt and water - how the diagram is built up, and how to interpret it. It includes a brief discussion of solubility curves. Solid-Liquid Phase Diagram of the Binary System ... Taking the phase-change temperature as the longitudinal coordinate and the mass fraction of C 18 -OH as the transverse coordinate, the solid-liquid phase diagram of the binary system (C 18 -acid + C 18 -OH) is shown in Figure 2. Figure 2 Phase diagram of the binary system (C 18 -OH + C 18 -acid). Phase Diagrams - Chemistry The temperature and pressure conditions at which a substance exists in solid, liquid, and gaseous states are summarized in a phase diagram for that substance. Phase diagrams are combined plots of three pressure-temperature equilibrium curves: solid-liquid, liquid-gas, and solid-gas.
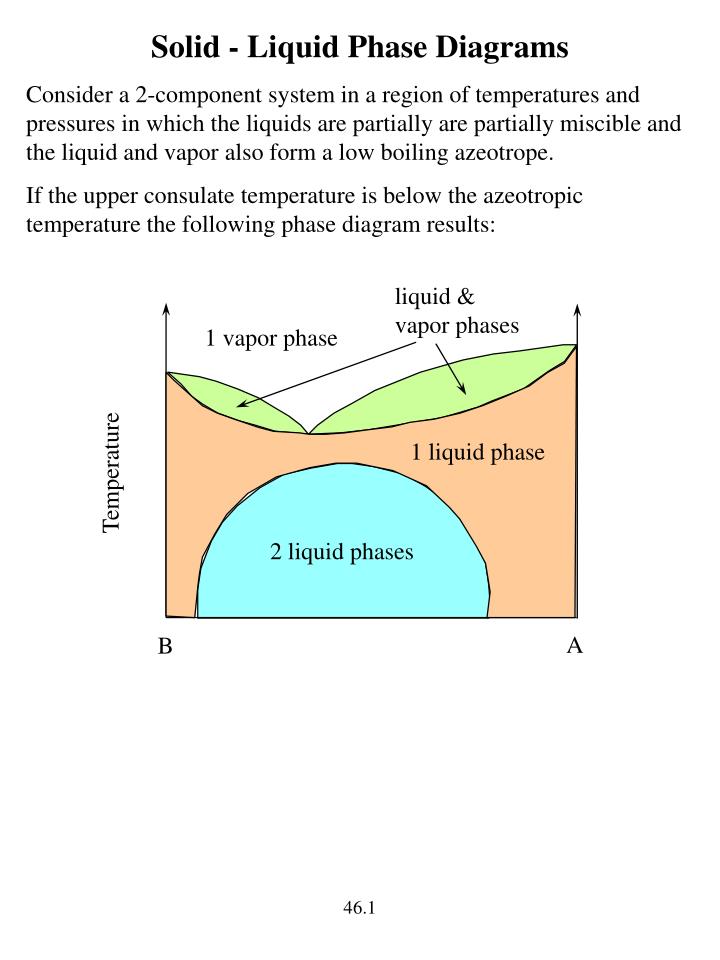
wiring schemas fr: Diagram Phase Of Water The Two Curves, Or Lines, Indicate The Areas Of Solid, Liquid And Gas. Phase diagram of water consists of three curves sublimation curve, evaporation curve and melting curve meeting each other at a point called triple point. For h2o melting point decreases with increasing pressure, for co2 melting point increases with increasing pressure. Solid Solution Phase Diagram - James Madison University The solid solution phase diagram explains the behavior of chemical solid solution series, such as the transition from high temperature, calcium-rich plagioclase to low temperature sodium-rich plagioclase, or the transition from high temperature magnesium-rich to low temperature iron-rich crystals in ferromagnesium minerals (e.g. olivine, pyroxene). Binary Solid-Liquid Phase Diagram | Chem Lab Introduction Solid-liquid phase diagrams show the phase relationships in mixtures of two or more components and are very important in understanding the behavior of mixtures in metallurgy, material science and geology. Solid Liquid Phase Diagram - Solid-Liquid Phase Diagram in ... Solid-Liquid Phase Diagram in a Two-Component System 1 Introduction The substances we encounter everyday are commonly mixtures of two or more components. For example, brass is a mixture of copper and zinc, and dish-washing detergent is a mixture of many chemicals. The components may interact with each other in a variety of different manners.
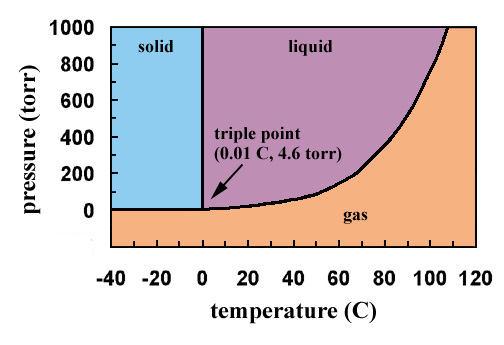
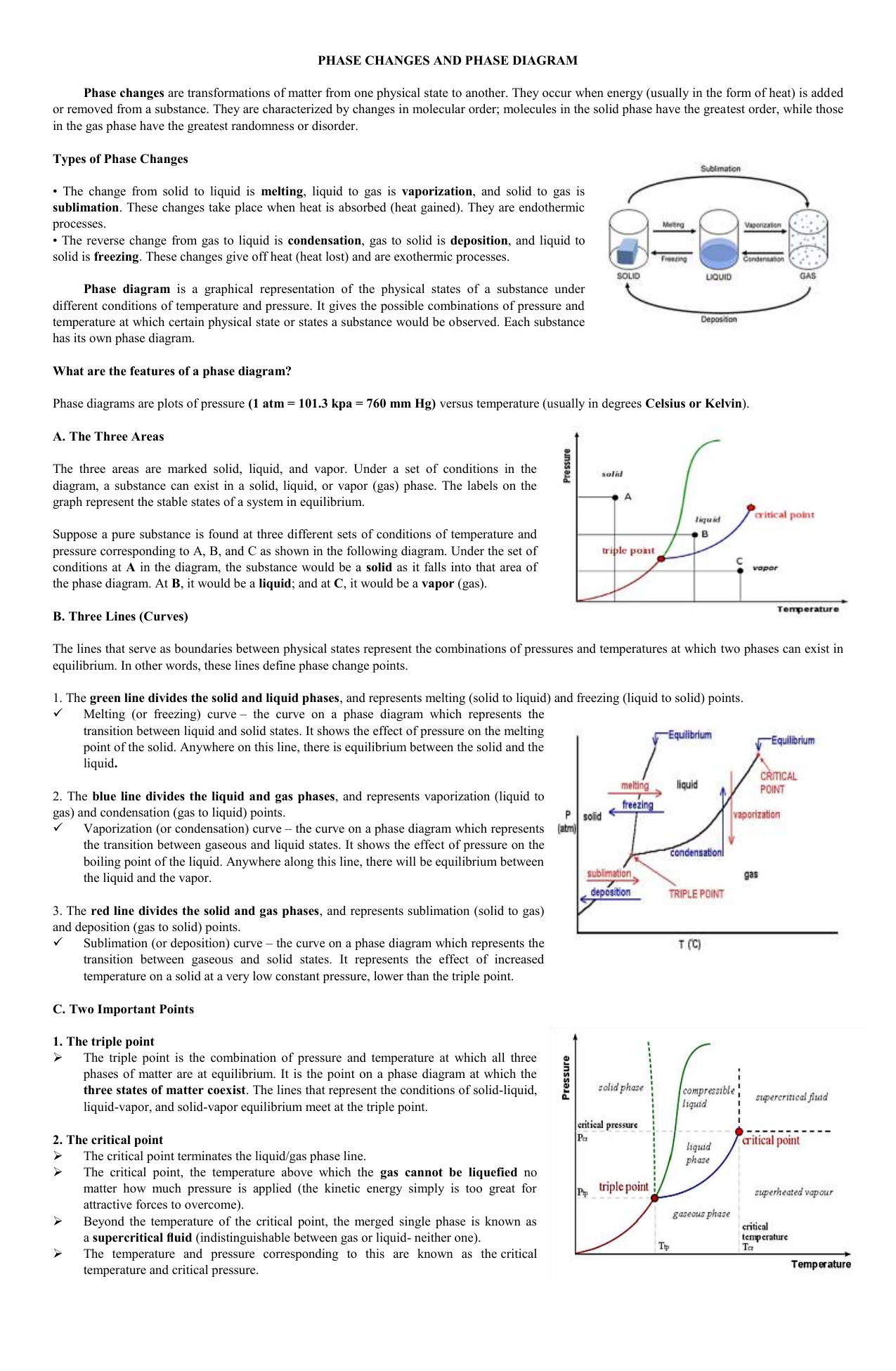
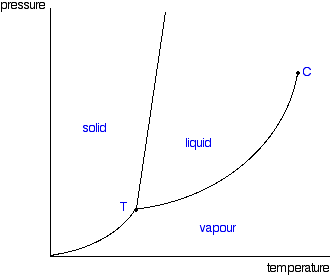
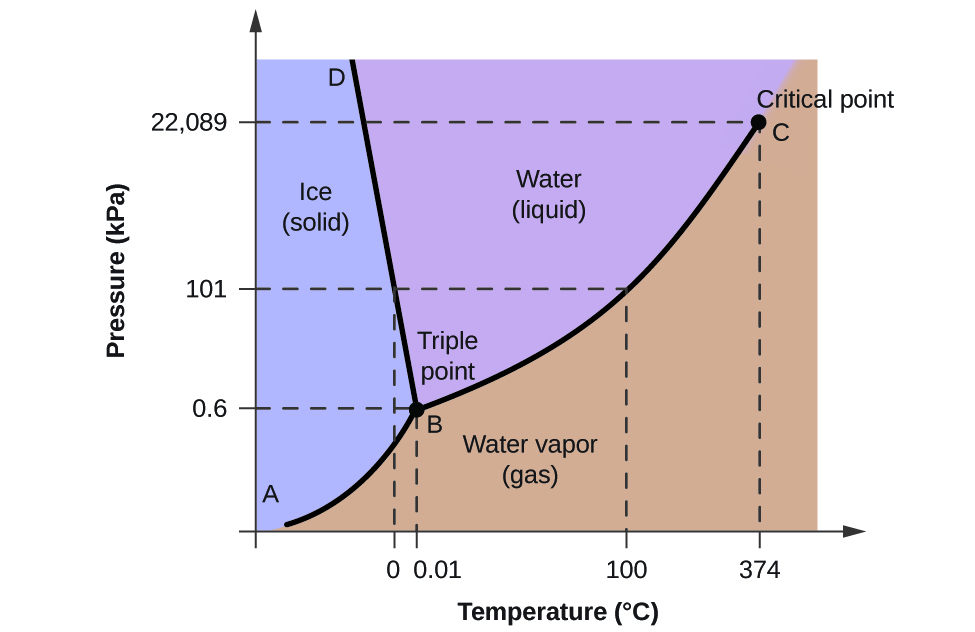
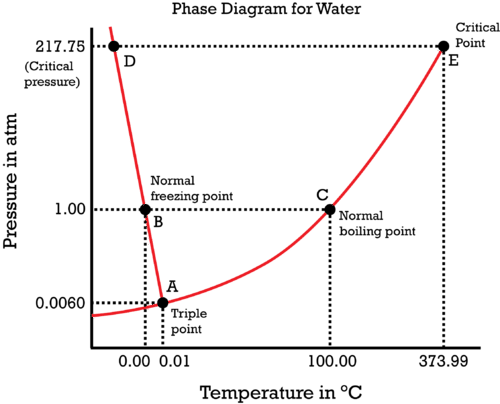
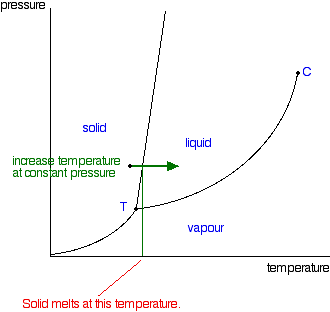
Phase Diagram of Carbon Dioxide - University of Manitoba A phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and vapor) of a substance can exist. Both phase diagrams for water and carbon dioxide have the same general . Y-shape, just shifted relative to one another. This shift occurs because the liquid phase in the dry ice can only occur at higher temperatures and pressures, whereas, … The Fe-FeSi phase diagram at Mercury's core conditions ... Literature on the Fe-Si system at high pressures report a series of miscibility gaps in the solid phase, between Si-poor hcp or fcc phases and an Si-rich B2 phase 26,28,38, or between the Si ... Phase (matter) - Wikipedia A typical phase diagram for a single-component material, exhibiting solid, liquid and gaseous phases. The solid green line shows the usual shape of the liquid-solid phase line. The dotted green line shows the anomalous behavior of water when the pressure increases. The triple point and the critical point are shown as red dots. Solid-liquid phase equilibrium and phase diagram of the ... The phase diagram belongs to a simple-type ternary system, and neither double salt nor solid solution was formed. Based on the phase diagrams of this system at 298.15 and 338.15 K, the cycle separation process for CsNO 3 recovery from the eluant of sodium nitrate mixture solution was evolved.
Phase Diagrams | Liquids and Solids - Nigerian Scholars A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance.
Phase Diagrams | Boundless Chemistry - Lumen Learning Phase diagrams are divided into three single phase regions that cover the pressure-temperature space over which the matter being evaluated exists: liquid, gaseous, and solid states. The lines that separate these single phase regions are known as phase boundaries.
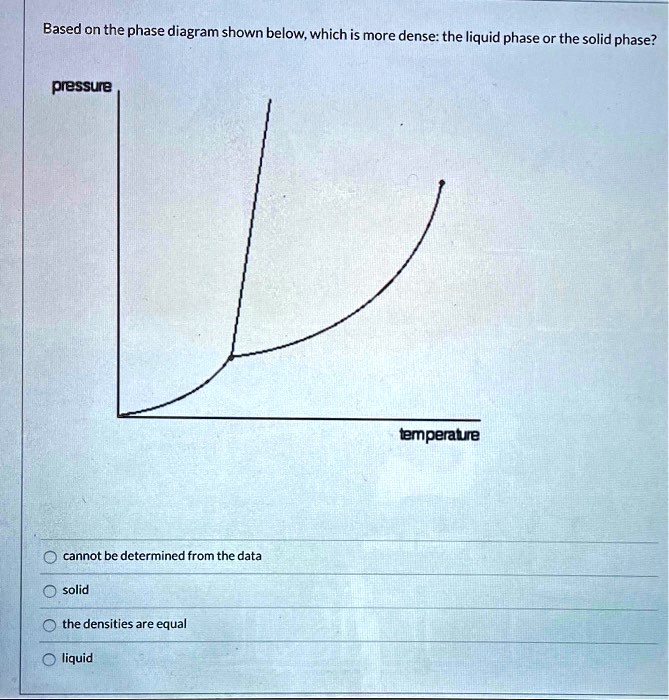
Phase diagram - Wikipedia For most substances, the solid-liquid phase boundary (or fusion curve) in the phase diagram has a positive slope so that the melting point increases with pressure. This is true whenever the solid phase is denser than the liquid phase.
PDF Solid - Liquid Phase Diagram of a Binary Mixture: The ... Experimental solid - liquid phase diagrams are constructed from observations of the cooling curves (temperature vstime) of molten mixtures through the point of solidification. When a pure liquid is cooled, the temperature may drop below the melting point without the formation of crystals - a phenomenon known as "supercooling".
Phase Diagrams | Dornshuld Phase diagrams give the state of a substance (solid, liquid, gas) given a specific set of conditions such as temperature and pressure. Below is an example of a simple phase diagram for water. 1 It is well known that water exists as a solid at or below its freezing point of 0 °C.
PDF Binary Solid-Liquid Phase Diagram Introduction remaining liquid solution. In the salt water analogy, the solid ice (pure H2O) is in equilibrium with the liquid H2O that remains in the unfrozen salt water. Mixtures of naphthalene and diphenylamine, both solids in the pure state at room temperature, will be prepared and their phase transitions studied by means of a thermal analysis.
PDF Determination of the Solid-Liquid Phase Diagram for ... • Phase Diagrams Phase diagrams are graphs that give information on the equilibrium temperature and pressure for a particular compound. The equilibria occur for the solid- liquid plateau, liquid-vapor plateau and solid-vapor plateau. In this experiment, the phase diagram is shown for the solid-liquid equilibrium point, and varies from 100% ...
PDF Chapter Outline: Phase Diagrams Isomorphous system -complete solid solubility of the two components (both in the liquid and solid phases). Binary Isomorphous Systems (I) Three phase region can be identified on the phase diagram: Liquid (L) , solid + liquid (α +L), solid (α ) Liquidus line separates liquid from liquid + solid Solidusline separates solid from liquid + solid α+ L α
Solid-Solid-Liquid Equilibrium - Wolfram Demonstrations ... Solid-liquid equilibrium and solid-solid-liquid equilibrium are both represented in the phase diagram. Mixtures of pure solids are immiscible. The relative amounts of the four possible phases, given the temperature and mole fraction of (represented by the black point in the - - diagram), are shown in the bar graph to the right of the diagram.
Solid-liquid phase diagrams - YouTube Solid-liquid phase diagrams
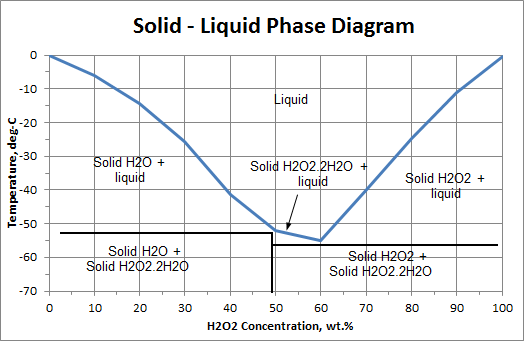
Solid-liquid Phase Diagram | USP Technologies Solid-liquid Phase Diagram . Ref: P.A. Giguere. "Complements au Nouveau Traite de C. himie Minerale - No. 4 - Peroxyde d'Hydrogene et Polyoxydes d'Hydrogene" Paris, Masson 1975 (181 p).
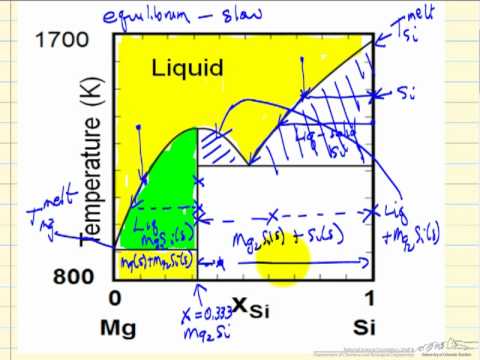
Solid-Liquid Phase Diagrams - YouTube Describes the regions of a liquid-solid, T-x phase diagram for a system composed of Mg and Si. Made by faculty at the University of Colorado Boulder Departme...
PDF Phase Diagrams, Solid Solutions, Phase Transformations Phase Diagrams: composition of phases At TA= 1320°C: Only Liquid (L) present CL= C0 ( = 35 wt% Ni) At TB= 1250°C: Both and L present At TD= 1190°C: Only Solid ( ) present C = C0( = 35 wt% Ni) C L = C liquidus ( = 32 wt% Ni) C = C solidus ( = 43 wt% Ni) 18 • Rule 3:If we know T and Co, then we know: --the amount of each phase (given in wt%).
PDF Simple Solid - Liquid Phase Equilibria An understanding of solid liquid phase equilibria is required to describe and calculate phase diagrams of interest in both fundamental materila science and applications such as crystallizer design. A very simple phase diagram is shown below. For this system the two solid phase do not mix (form intercallated crystals) or form solid compounds.
PDF Experiment 1 Solid - Liquid Phase Diagram The binary solid-liquid phase diagram for the naphthalene-diphenylamine system will be constructed from cooling curves. Several mixtures of different ratios of the two components will be melted, and temperature versus time curves will be plotted as the mixtures cool.
















:max_bytes(150000):strip_icc()/phase_diagram_generic-56a12a1b5f9b58b7d0bca817.png)

0 Response to "36 solid liquid phase diagram"
Post a Comment