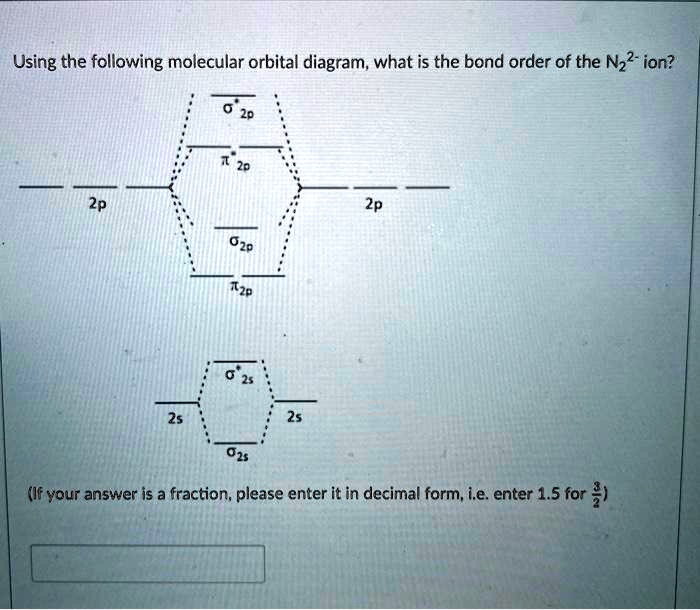
36 n22- molecular orbital diagram
MO Diagram for N2+ (Molecular Orbital) - YouTube There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start... Molecular Orbital MO Diagram for N2(2-) - YouTube the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ...
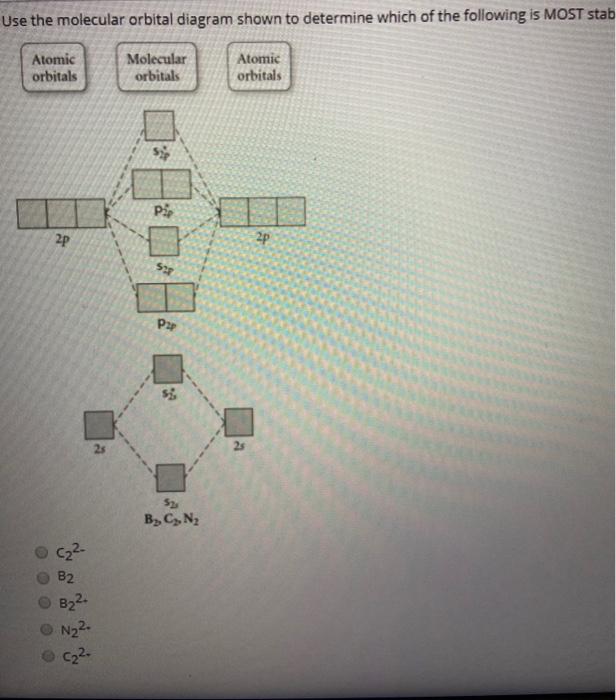
What Is The Molecular Orbital Diagram For B2 ... C22- Molecular Orbital Diagram. N22+ B22+ B B2 CeV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of the lowest energy molecular orbital with respect to the 3s orbital of sulfur. This lowest energy ...
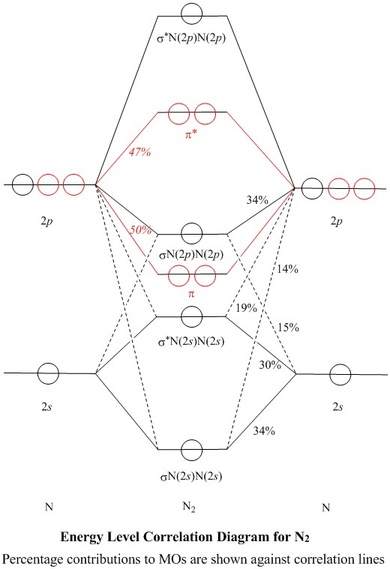
N22- molecular orbital diagram
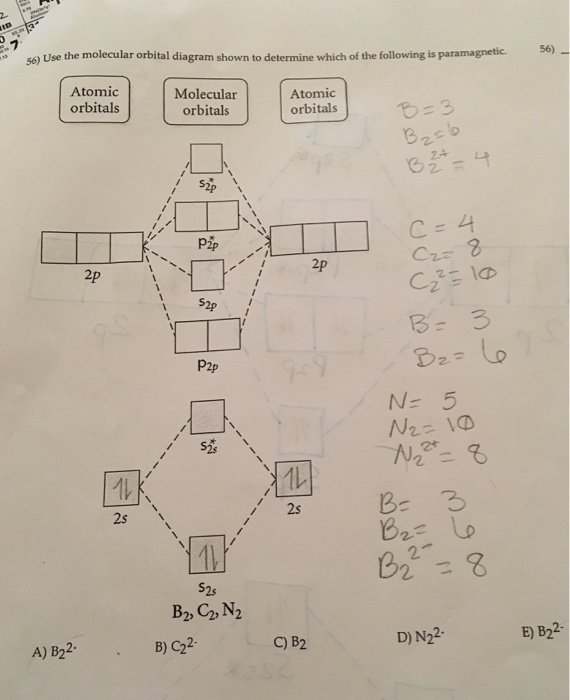
Answered: Draw the molecular orbital diagram… | bartleby ASK AN EXPERT. ASK. Science Chemistry Q&A Library Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+. Based on molecular orbital theory, the bond order of the N ... Scientists of the 19th Century found that the equation, 1/l = RH(1/n21 - 1/n22) (where RH = 1.097 ´ 107 m-1, and n1 and n2 are whole numbers) predicts the wavelength of the lines in the emission spectrum of the H atom. ... combination of two 2p atomic orbitals may give rise to either σ or π type molecular orbitals. asked Sep 3, 2019 in ... PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
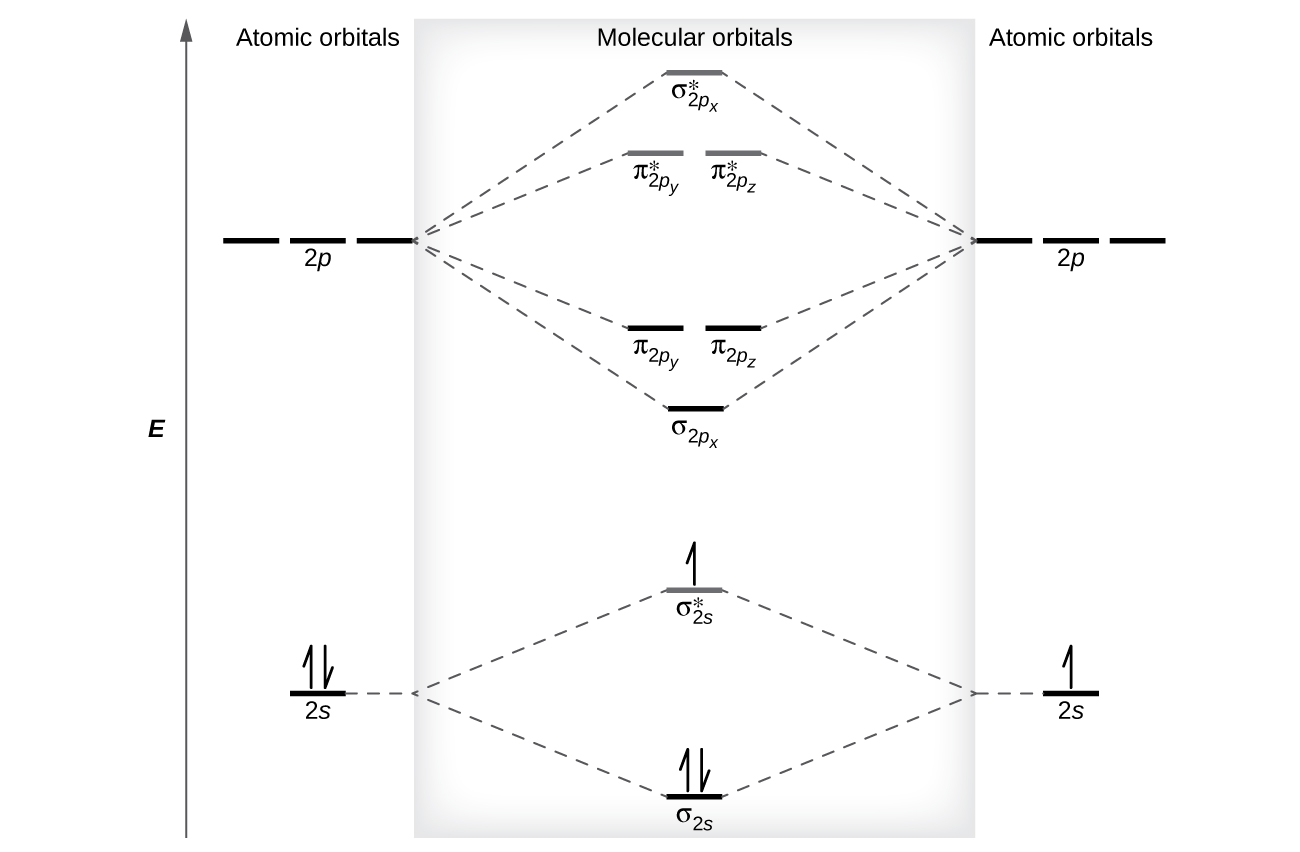
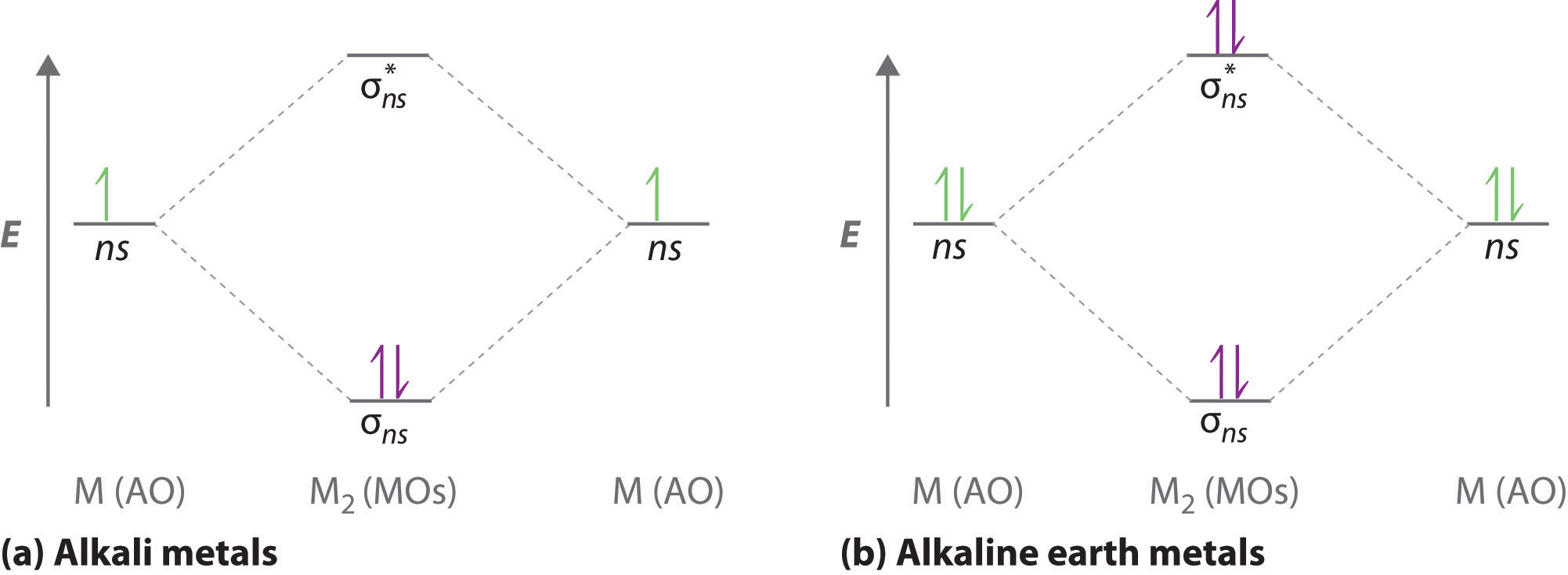
N22- molecular orbital diagram. Draw the molecular orbital diagram of N2N2 + N2 Write ... This picture shows the molecular orbital diagram of N 2 − . Orbitals represented by ∗ are antibonding orbitals and the orbitals without ∗ are bonding orbitals. Bond order can be calculated by the formula: Bond order = bonding electrons - antibonding electrons 2 What is the bond order of N2+, N2-, and N22-? | Study.com Bond Order: Bond order is a measurement of electrons that participate in bond formation. It shows a chemical bond is stable. If the value of a bond order is high, the atom contains a strong bond. Energy level diagram for Molecular orbitals - Chemical ... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ... 8.4 Molecular Orbital Theory - Chemistry Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
Test: Molecular Orbital Theory | 23 Questions MCQ Test ... Test: Molecular Orbital Theory. QUESTION: 1. Direction (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct. Q. Assuming that Hund's rule is violated, the bond order and magnetic nature of the diatomic molecule B2 is. Solved The molecular orbital diagrams for the N22-and 0,2 ... The molecular orbital diagrams for the N22-and 0,2- anions are given below. What can be said about which is predicted to be more stable? N- N, 2- N- 0- 0" + 11 11 * 14 11 2p 2th 1 11 11 11 11 11 11 11 25 11 O The bond orders are the same so both are equally stable. O 022-has a bond order of 1 and is more stable than N2+. What is the molecular orbital diagram for C_2^-? | Socratic The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be. how to draw molecular orbital diagram of n2 - We Had A Big ... In the molecular orbital diagram for the molecular ion N 2 the number of electrons in the σ 2p molecular orbital is. Bond order 3. The diagram above is the molecularN2 molecular orbital energy level diagram picture is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a ...
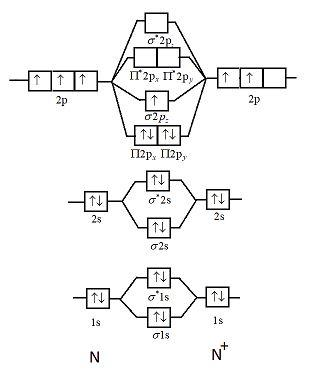
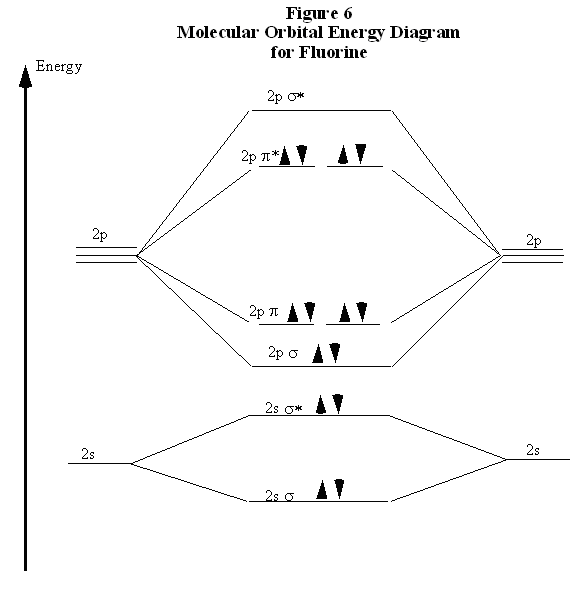
N2+ Mo Diagram - schematron.org This is because, according to molecular orbital theory , it has fewer electrons in bonding orbitals. The diagram above is the molecular.N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web ... With the help of molecular orbital diagram show that Ne2 ... The energy of σ2pz molecular orbital is greater than π2px and π2py molecular orbitals in nitrogen molecule. asked Aug 22, 2018 in Chemistry by Sagarmatha ( 54.6k points) chemical bonding What are the molecular orbital configurations for N_2^+, N ... If we build the MO diagram for "N"_2, it looks like this: First though, notice that the p orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be. Anyways, for the electron configurations, you would use a notation like the above. g means "gerade", or even symmetry upon inversion, and u means "ungerade", or odd symmetry upon inversion. draw the molecular orbital diagram of n2 also find its ... Write the molecular orbital diagram of N2+ and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric.
is b22+ paramagnetic or diamagnetic Use the molecular orbital diagram shown to determine which of the following are paramagnetic. Device self test is a Admin cmd, which is not supported by inbox driver API. Nitrogen (N$_2$) is not paramagnetic but diamagnetic. paramagnetic. list of dog breeders in va, Dogs: The Ultimate Guide to Over 1,000 Dog Breeds.
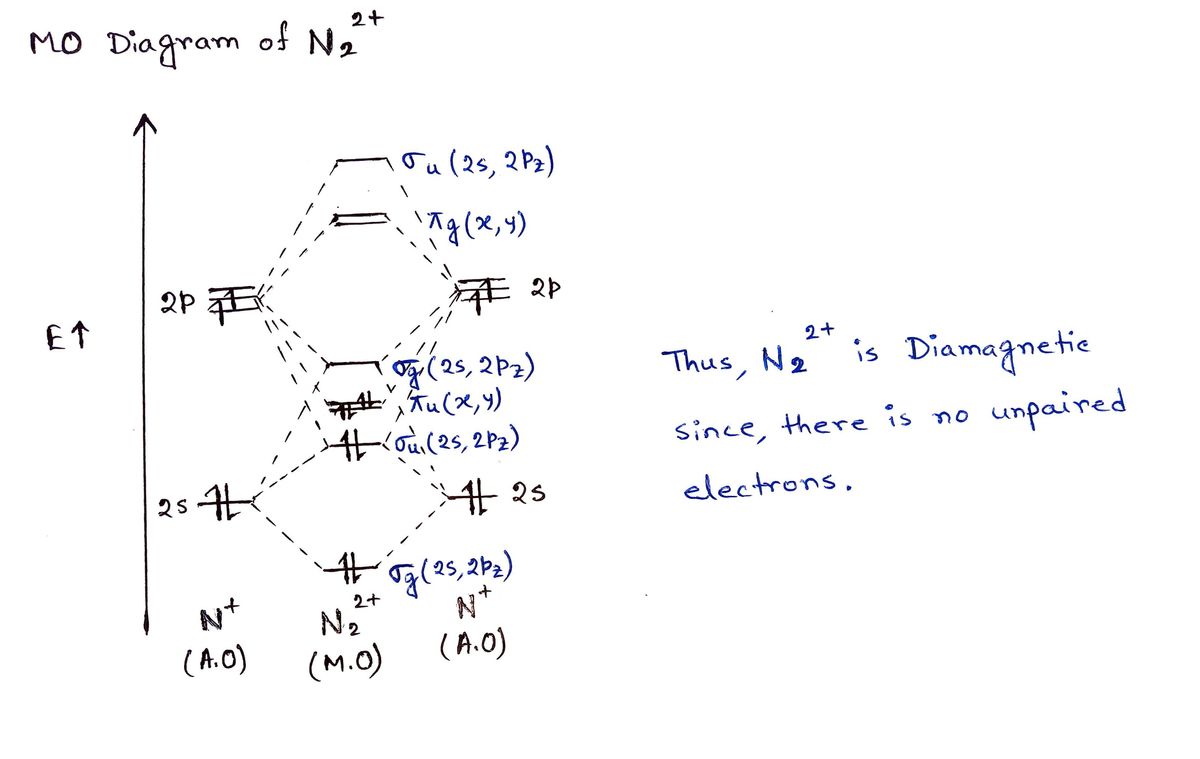
write the molecular orbital diagram of n2 and calculate ... Write the molecular orbital diagram of N2+ and calculate their bond order. Asked by sonkarshiva009 | 13th Mar, 2019, 05:47: PM. Expert Answer: Electronic configuration of N-atom(Z=7) is ...
Engineering Chemistry Quiz with Solution Topic Molecular ... Engineering Chemistry Quiz with Solution Topic Molecular Orbital Theory. 1. Arrange the following molecules in the order of increasing stability. Explanation: The order of stability is directly proportional to the bond order. Therefore, the correct order of stability is N22- < N2- = N2+ < N2.
Leave a Comment Cancel reply - BYJUS Draw the molecular orbital diagram of N2 and calculate the bond order. Answer: The bond order shows the number of chemical bonds present between a pair of atoms. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Bond order formula is given as below.
Use the molecular orbital diagram shown to determine which ... The term paramagnetism depends upon the number of unpaired electrons. More the number of unpaired electrons, more will be the paramagnetism.The given molecular orbital diagram contains orbitals to be filled in the order: s1s, s*1s, s2s, s*2s, p2p, s2p, p*2p, s*2pAccording to molecular orbital theory, The electronic configuration of C22-= s1s2, s*1s2, s2s2, s*2s2, p2p4, s2p2=> Number of ...
C22- Molecular Orbital Diagram N22+ B22+ B B2 CeV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of the lowest energy molecular orbital with respect to the 3s orbital of sulfur. This lowest energy orbital is .
Draw the molecular orbital diagram of N2 , N2^ + N2 ... Click here👆to get an answer to your question ️ Draw the molecular orbital diagram of N2 , N2^ + N2 ^ - . Write their electronic configuration, find the bond order and predict their magnetic behaviour. Arrange the above in increasing order of bond length.
electronic configuration - Molecular orbital (MO) diagram ... Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion. 6. What is the correct molecular orbital diagram for the d orbitals in platinum for the tetraammineplatinum(II) complex? 1. Explanation of the missing 1-s orbital electrons of carbon in the molecular orbital diagram of methane.
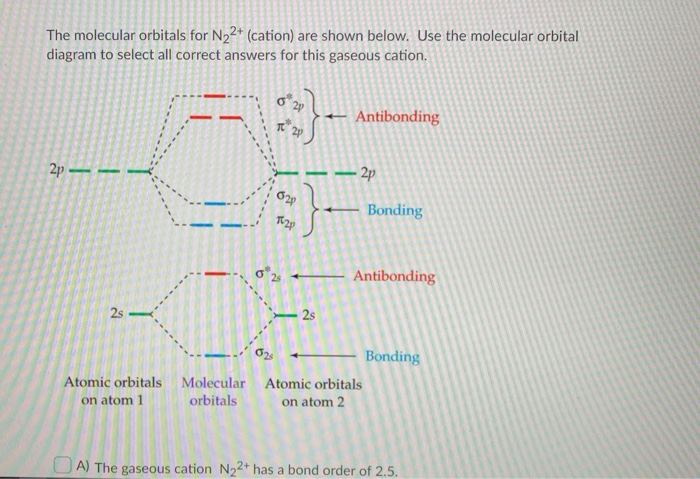
Solved Use Molecular Orbital (MO) diagrams to rank N22 ... Answer to Solved Use Molecular Orbital (MO) diagrams to rank N22+, N2,
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Based on molecular orbital theory, the bond order of the N ... Scientists of the 19th Century found that the equation, 1/l = RH(1/n21 - 1/n22) (where RH = 1.097 ´ 107 m-1, and n1 and n2 are whole numbers) predicts the wavelength of the lines in the emission spectrum of the H atom. ... combination of two 2p atomic orbitals may give rise to either σ or π type molecular orbitals. asked Sep 3, 2019 in ...
Answered: Draw the molecular orbital diagram… | bartleby ASK AN EXPERT. ASK. Science Chemistry Q&A Library Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+.



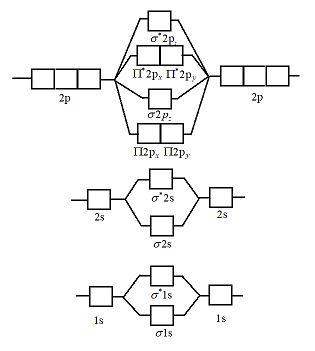
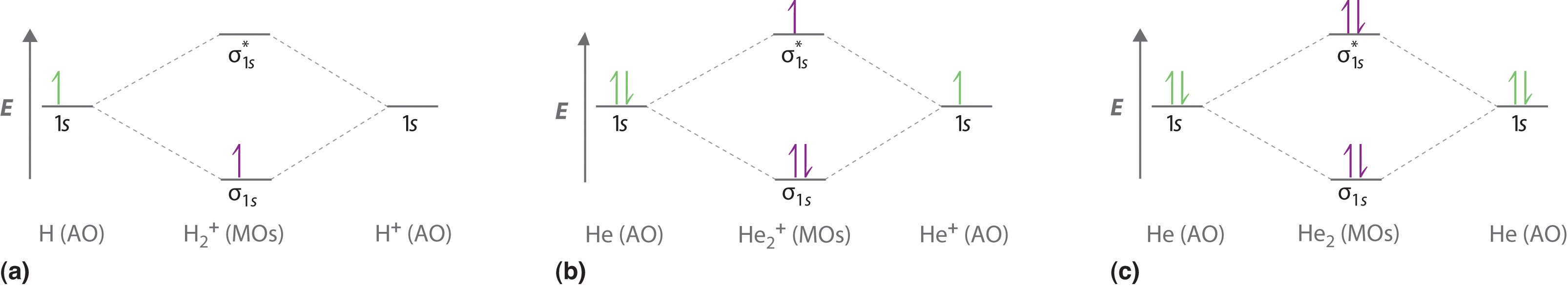











0 Response to "36 n22- molecular orbital diagram"
Post a Comment