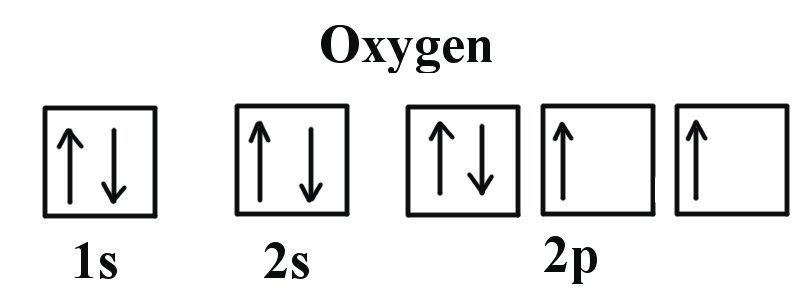
40 orbital diagram for oxygen
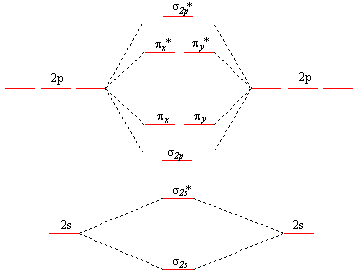
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... - Oxygen - hydrogen interactions share 2 electron Æ H ─ O. - Oxygen also has two lone pairs. • Energy level diagram represents this interaction. - Two s orbitals interaction to create a low energy bonding and high energy anti-bonding molecular orbital. PDF Figure 9.32: The molecular orbital energy-level diagram for Figure 9.40: When liquid oxygen is poured into the space between the poles of a strong magnet, it remains there until it boils away. This attraction of liquid oxygen for the magnetic field demonstrates the paramagnetism of the O2 molecule. Figure 9.41: The molecular orbital energy-level diagram for...
valenceelectrons.com › oxygen-electron-configurationOxygen(O) electron configuration and orbital diagram Oxygen(O) is the 8th element in the periodic table and its symbol is ‘O’. This article gives an idea about the electron configuration of oxygen and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles.
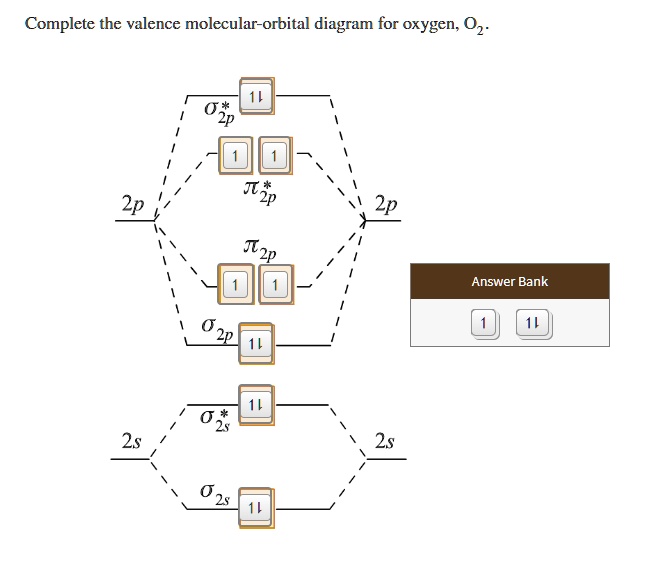
Orbital diagram for oxygen
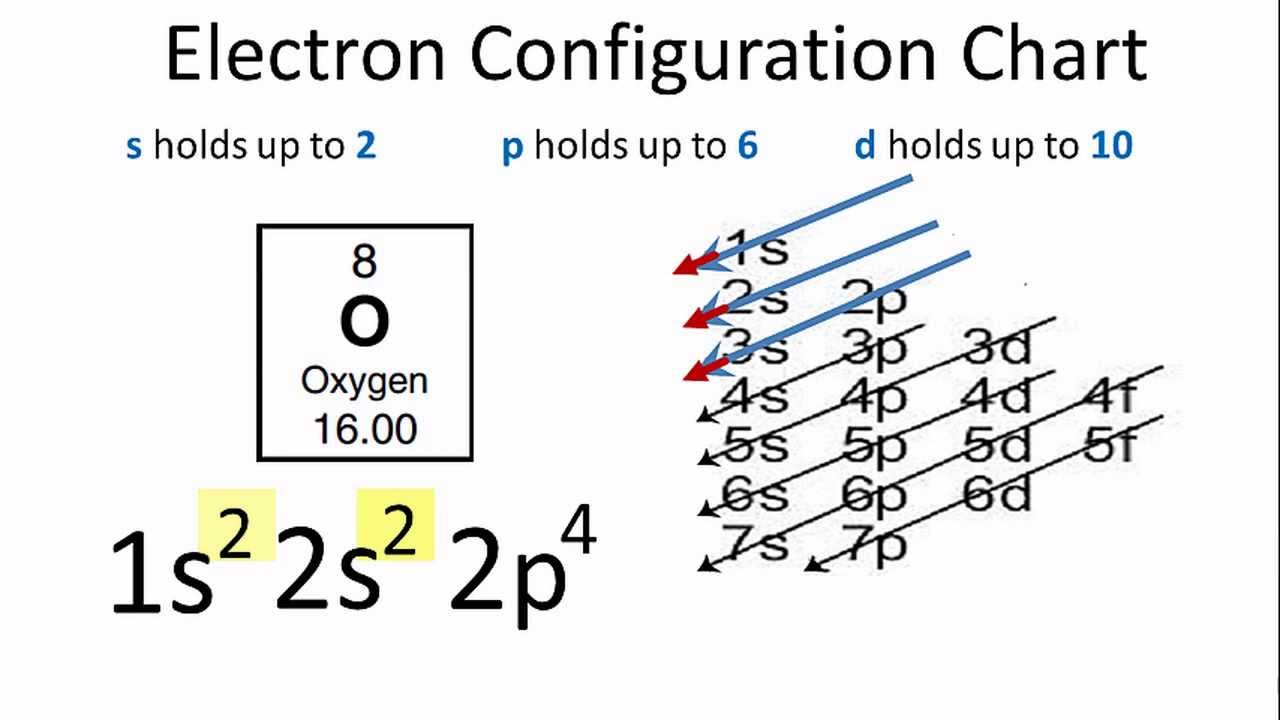
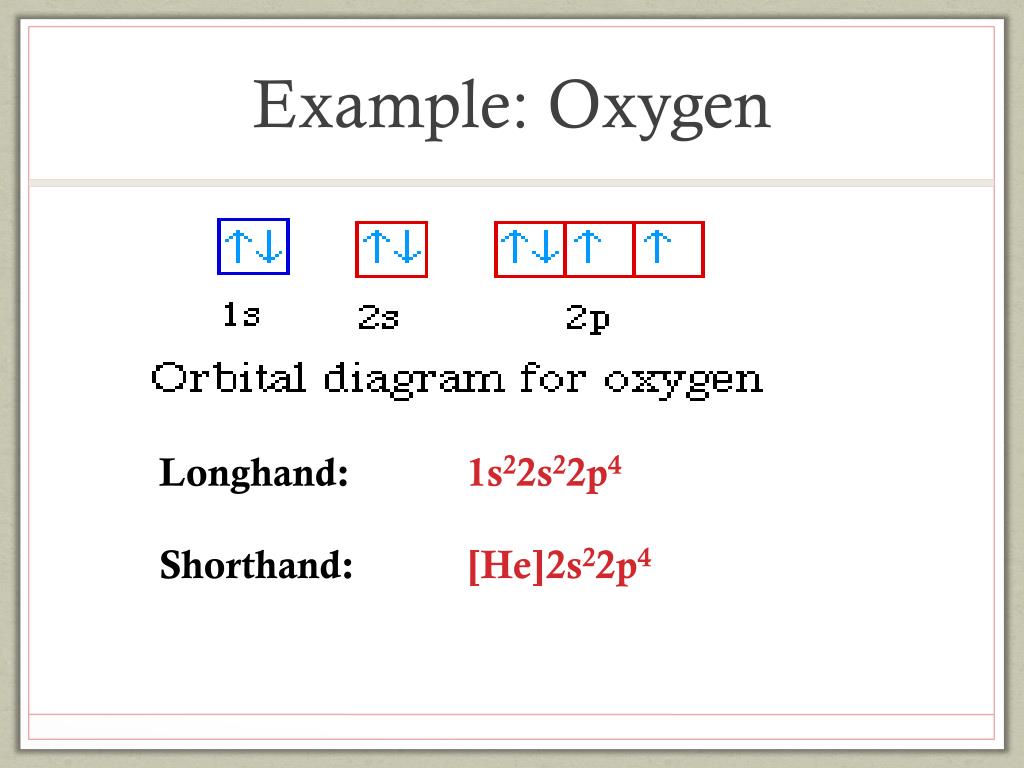
Electron configuration for Oxygen (element 8). Orbital diagram O (Oxygen) is an element with position number 8 in the periodic table. Located in the II period. Melting point: -218.4 ℃. Density: 0.00133 g/cm3. Below is the electronic diagram of the Oxygen atom. Distribution of electrons over energy levels in the O atom 1-st level (K): 2 2-st level (L): 6. en.wikipedia.org › wiki › Molecular_orbital_diagramMolecular orbital diagram - Wikipedia Carbon and each oxygen atom will have a 2s atomic orbital and a 2p atomic orbital, where the p orbital is divided into p x, p y, and p z. With these derived atomic orbitals, symmetry labels are deduced with respect to rotation about the principal axis which generates a phase change, pi bond ( π ) [26] or generates no phase change, known as a ... Orbital Diagram for Oxygen Orbital Diagram for Oxygen. ●Abbreviated Electron Configuration of Oxygen(O) Step-1: To write electron configuration of oxygen(O),we have to know the atomic number of oxygen.The atomic number of carbon is 8.So oxygen has 8 electrons and 8 protons.
Orbital diagram for oxygen. Orbital Energy Diagram For Oxygen - Free Catalogs A to Z Oxygen(O) Electron Configuration With Full Orbital Diagram. 9 hours ago Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. Oxygen Definition, Facts, Symbol, Discovery, Property, Uses - - Orbital Diagram for Oxygen. Atomic Data of Oxygen (Element 8). Oxygen gas is commercially used in the steel industry for removing undesirable compounds and impurities during the forging process [1, 12]. Oxygen orbital diagram - Big Chemical Encyclopedia Figure 1.7 Molecular orbital diagram for molecular oxygen, O2. From K. M. Ralls, T. H. Courtney, and J. Wulff, Introduction to Materials Science and Engineering. Copyright 1976 by John Wiley Sons, Inc. This material is used by permission of John Wiley Sons, Inc. Molecular Orbital diagram for the molecule, oxygen, O2. - YouTube This video shows the construction of a molecular orbital (MO) diagram for the diatomic molecule, O2, using the valence electrons of each oxygen.
Orbital Diagrams Chemistry Tutorial Orbital Diagrams for Period 3 Elements. The electronic configuration of atoms of all Period 3 elements begins with a completed 1st and 2nd energy level (filled K Worked Example of an Orbital Diagram Problem. Question 1: The ground state electronic configuration for an atom of oxygen is 1s2 2s2 2p4. Molecular Orbital Theory | Chemistry: Atoms First Example 2: Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons. Draw the molecular orbital diagram for the oxygen molecule, O2. We draw a molecular orbital energy diagram similar to that shown in Figure 12. Each oxygen atom contributes six electrons, so the... Oxygen Electron Configuration (O) with Orbital Diagram Oxygen Electron Configuration: O is an odourless, colorless, reactive gas. Its atomic number is 8 and it the life-supporting component of the air. There is Six valence electron in the Oxygen. You can see the above Orbital Dot Diagram. The symbol of Oxygen is O and the atomic Mass of Oxygen is 16. 8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons. Draw the molecular orbital diagram for the oxygen molecule, O2. We draw a molecular orbital energy diagram similar to that shown in Figure 8.37. Each oxygen atom contributes six electrons, so the diagram appears as...
Atomic Orbital Diagram for Oxygen | Online Chemistry Help The above diagram explains the molecular orbital energy level diagram for molecules of Oxygen and other heavier elements. The electronic configuration of oxygen (Z=8) in the ground state is 1s22s22p4. Each oxygen atom has 8 electrons; hence in O22 is as follows molecule there are 16... PDF Electron Configuration Example Script The last example is an orbital diagram. We are going to draw the orbital diagram for oxygen. A neutral oxygen atom has 8 electrons, so the Each filled orbital is assigned two electrons of opposite spin according to the Pauli Exclusion Principle. So two of oxygen's electrons go in the 1s orbital, and... What is the molecular orbital diagram for oxygen? - Quora From the molecular orbital diagram, we observe that oxygen has two unpaired electrons which is consist with the paramagnetic nature of oxygen. The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in... How to Do Orbital Diagrams | Sciencing Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy Dot diagrams are very different to orbital diagrams, but they're still very easy to understand. They consist of the symbol for the element in the...
How do yo write the orbital diagram for oxygen? | Socratic The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps!
periodictableguide.com › orbital-diagram-of-allOrbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
Nitrogen Bohr Model - How to draw Bohr diagram for ... Electron dot diagram of a Nitrogen atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Nitrogen, we got to know, it has 5 valence electrons. So, just represent these 5 …
terpconnect.umd.edu › ~wbreslyn › chemistryElectron Configuration for Phosphorus (P) - UMD In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
9.6: Quantum-Mechanical Orbitals and Electron Configurations An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a Draw the orbital filling diagram for carbon and write its electron configuration.
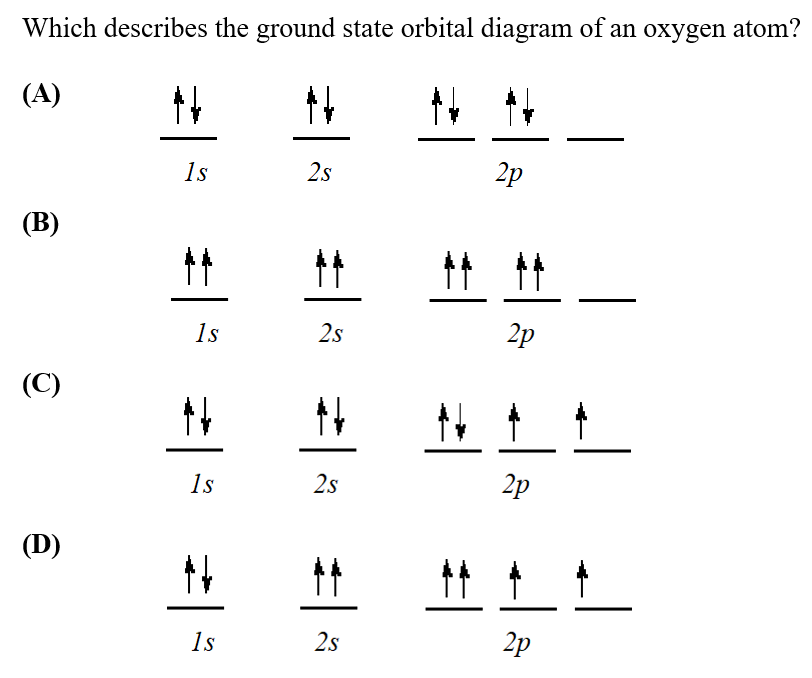
Welcome to CK-12 Foundation | CK-12 Foundation The orbital diagram on the left is the correct orbital diagram, because it obeys Hund's Rule, meaning that there is less electron-electron repulsion and, as a result, the electrons have lower energies (remember Draw the orbital diagram for oxygen, . Use it to answer the following questions
Atomic and Molecular Orbital Diagram for Oxygen/O2 Molecular Orbital Theory Jaclyn & Simran PLTL Week 13 Enery Diagrams (atomic Vs Molecular Orbital) Atomic Orbital Configuration - 1, 2, 2, 3, 3, 4, 3, 4, 5, 4, 5, 6, 4 Remembering how to draw the MO diagram for B,C,N Vs O,F,Ne - using dry erase board, generic diagram handout, or other.
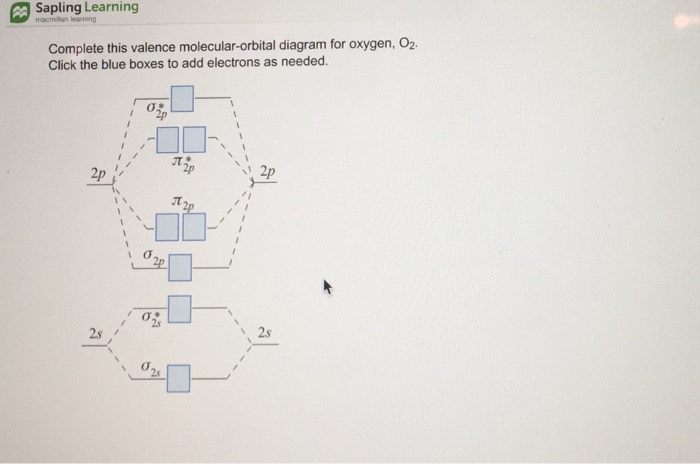
Complete This Valence Molecular Orbital Diagram For Oxygen O2 Looking at atomic oxygen we see there are a total of eight electrons so when we form a diatomic. By looking at the orbital diagram we can s...
13 Molecular orbital diagram of oxygen molecule. Reproduced from... Download scientific diagram | 13 Molecular orbital diagram of oxygen molecule. The oxygen reduction reaction (ORR) process in the cathode compartment occurs by the direct transfer pathway of 4-electrons from O2 to H2O (O2 + 2H2O + 4e − → 4 OH − , E0′ = 0.815 V, pH = 7) [30].
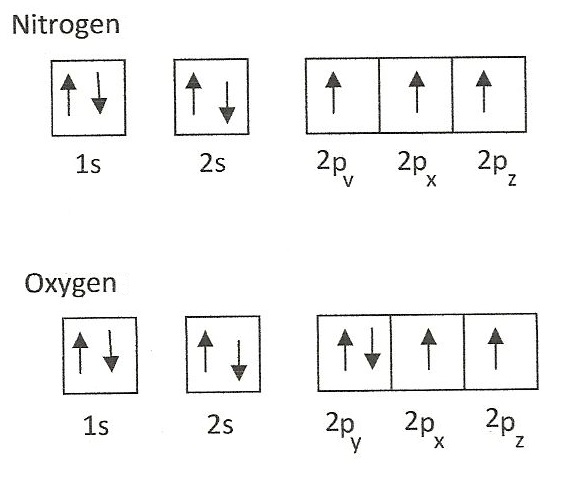
Nitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen (N) Nitrogen(N) excited state electron configuration. Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of nitrogen is 1s 2 2s 2 2p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Each sub-orbital can have a ...
opentextbc.ca › 8-4-molecular-orbital-theory8.4 Molecular Orbital Theory – Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
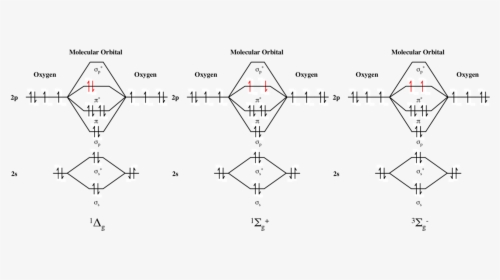
en.wikipedia.org › wiki › Singlet_oxygenSinglet oxygen - Wikipedia This more stable of the two excited states has its two valence electrons spin-paired in one π* orbital while the second π* orbital is empty. This state is referred to by the title term, singlet oxygen , commonly abbreviated 1 O 2 , to distinguish it from the triplet ground state molecule, 3 O 2 .
Orbital Diagrams — Overview & Examples - Expii An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals. The Basics of Orbital Diagrams. There are different types of orbitals, that all have different energy levels. These orbitals are filled with electrons (the...
Orbital Diagrams & Electron Configurations for Atoms and Ions Draw an orbital diagram for beryllium (Z=4) 1s Guidelines for drawing orbital diagrams 1s 1. Fill orbitals in order of increasing energy. 1s 2s 7 N nitrogen 14.01 2p Ti 8 O oxygen 16.00 Orbital diagrams for ions • anion (negative charge): ADD appropriate number of electrons • cation (positive)...
Molecular Orbital Theory This diagram suggests that the energy of an H2 molecule is lower than that of a pair of isolated atoms. The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.
chem.libretexts.org › Bookshelves › General_Chemistry9.8: Molecular Orbital Theory - Chemistry LibreTexts Feb 20, 2022 · The lithium 1s orbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.
PDF Electron Configurations | Orbital diagram for ground state 7. Consider the orbital diagram for oxygen in Model 2. b. Based on its position in the periodic table, explain how you know that your answer to part a is the correct number of electrons for oxygen.
Orbital Diagram for Oxygen Orbital Diagram for Oxygen. ●Abbreviated Electron Configuration of Oxygen(O) Step-1: To write electron configuration of oxygen(O),we have to know the atomic number of oxygen.The atomic number of carbon is 8.So oxygen has 8 electrons and 8 protons.
en.wikipedia.org › wiki › Molecular_orbital_diagramMolecular orbital diagram - Wikipedia Carbon and each oxygen atom will have a 2s atomic orbital and a 2p atomic orbital, where the p orbital is divided into p x, p y, and p z. With these derived atomic orbitals, symmetry labels are deduced with respect to rotation about the principal axis which generates a phase change, pi bond ( π ) [26] or generates no phase change, known as a ...
Electron configuration for Oxygen (element 8). Orbital diagram O (Oxygen) is an element with position number 8 in the periodic table. Located in the II period. Melting point: -218.4 ℃. Density: 0.00133 g/cm3. Below is the electronic diagram of the Oxygen atom. Distribution of electrons over energy levels in the O atom 1-st level (K): 2 2-st level (L): 6.



























0 Response to "40 orbital diagram for oxygen"
Post a Comment