40 gold foil experiment diagram
Rutherford Model of an Atom - Class 9, Structure of an atom Experimental set Up (1) He selected a thin gold foil. (2) The fast moving alpha particles are allowed to strike a very thin gold foil in vacuum. Observation (1) Most of the alpha particles pass straight through the gold foil without any deflection from their original path. Cell Cycle: Definition, Phases, and Diagram - Science Facts 18.01.2021 · The cell cycle occurs in an orderly and natural manner. A group of proteins called regulatory proteins ensures an error-free process. There are some checkpoints whose purpose is to control the system and determine whether the cell …
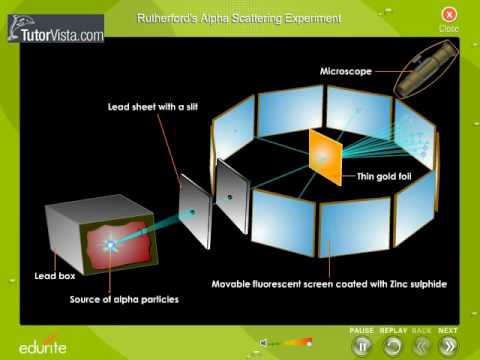
Explain Rutherfords alpha ray scattering experiment class ... Complete step by step answer: Diagram of Rutherford's α rays scattering experiment. Rutherford used the observations of Thomson's experiment to propose the atomic structure. Rutherford conducted the experiment using radioactivity phenomenon. He used radium bromide, RaBr which is a radioactive material. This substance emits α
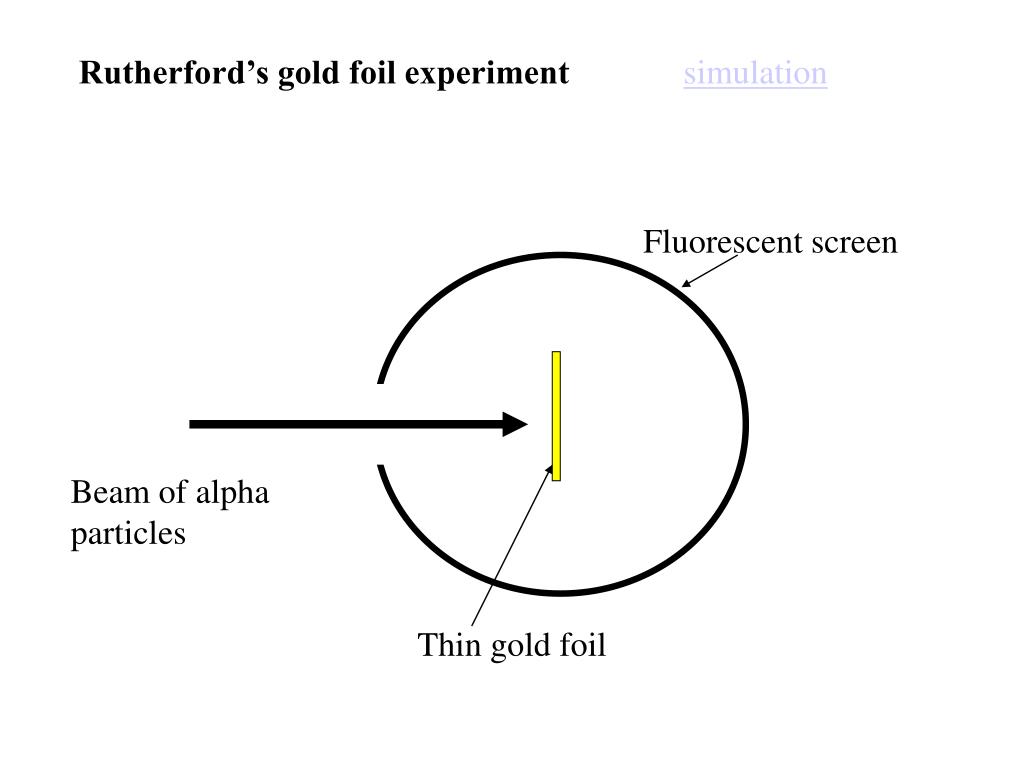
Gold foil experiment diagram
Gold Foil Experiment- Video quiz. Diagram | Quizlet Diagrams Flashcards Mobile Help Sign up Help Center Honor Code Community Guidelines Students Teachers About Company Press Careers Advertise Privacy Terms Follow us Language DeutschEnglish (UK)English (USA)EspañolFrançais (FR)Français (QC/CA)Bahasa IndonesiaItalianoNederlandspolskiPortuguês (BR)РусскийTürkçeTiếng Việt한국어中文 (简体)中文 (繁體)日本語 Exploring atoms: atom structure - Scootle See how scientists such as Ernest Rutherford have investigated the structure of atoms. Explore possible models. Fire charged particles at atoms and find which model best fits the results. This learning object is one in a series of six objects. Three of the objects are also packaged as a combined learning object. how to draw rutherford scattering experiment| how to draw ... Title: how to draw rutherford scattering experiment| how to draw diagram of rutherford gold foil experiment | rutherford alpha particle scattering experiment...
Gold foil experiment diagram. Geiger-Marsden experiments - Wikipedia The Geiger-Marsden experiments (also called the Rutherford gold foil experiment) were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. About Rutherford's Gold Foil Experiment - Sciencing About Rutherford's Gold Foil Experiment | Sciencing Ernest Rutherford, originally from New Zealand, is credited as being the father of nuclear physics for his discoveries in atomic structure, even though Hantaro Nagaoka, a physicist from the Imperial University of Tokyo, first proposed the theory of the nucleus as it is known today. What do you think would be the observation if the ... - Toppr If α-particle scattering experiment is carried out using a foil of any metal as thin as gold foil used by Rutherford, there would be no change in observations.But since other metals are not so malleable so, such a thin foil is difficult to obtain. If we use a thick foil, then more α-particles would rebound. Rutherford Model of the Atom: Definition & Diagram - Video ... 24.09.2021 · This diagram depicts the expected and the actual results of the gold foil experiment. The diagram on the left shows particles passing through the positively charged matrix of the plum pudding ...
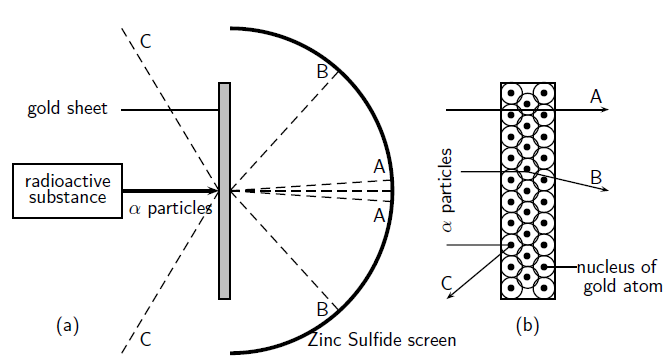
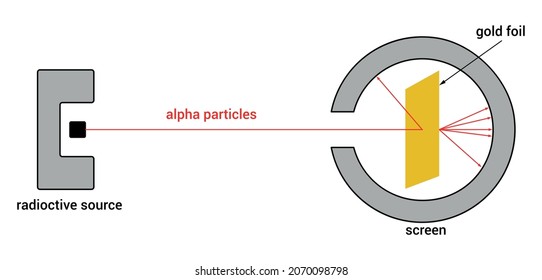
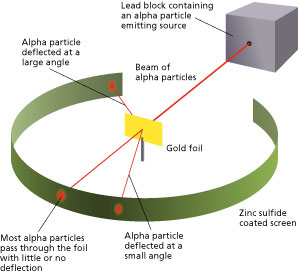
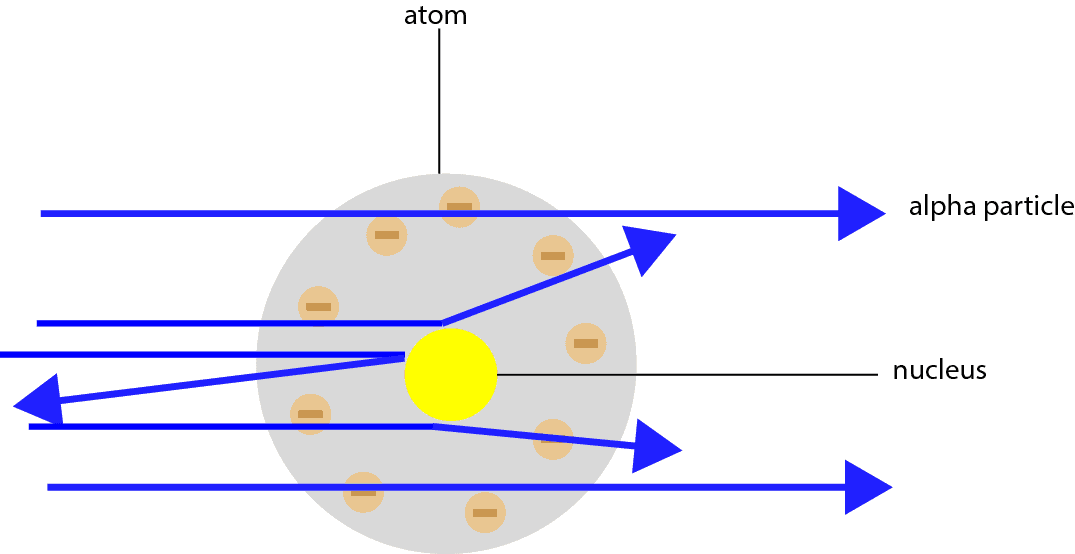
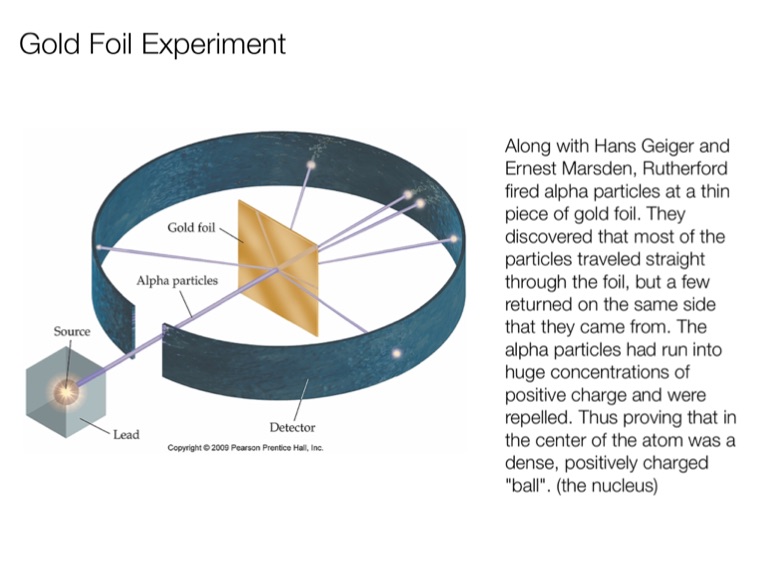
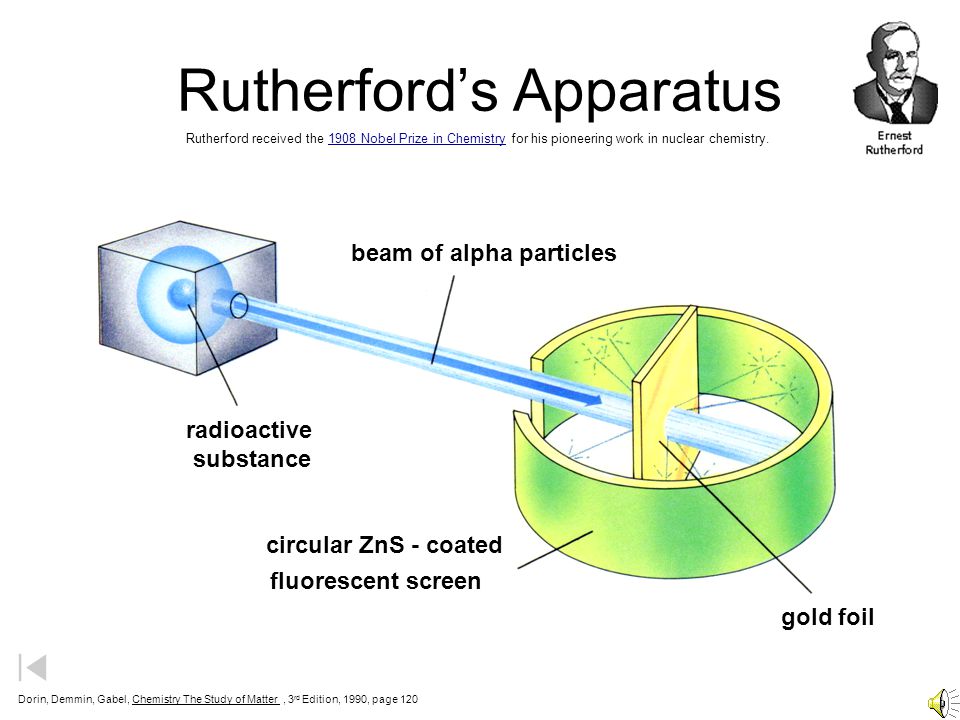
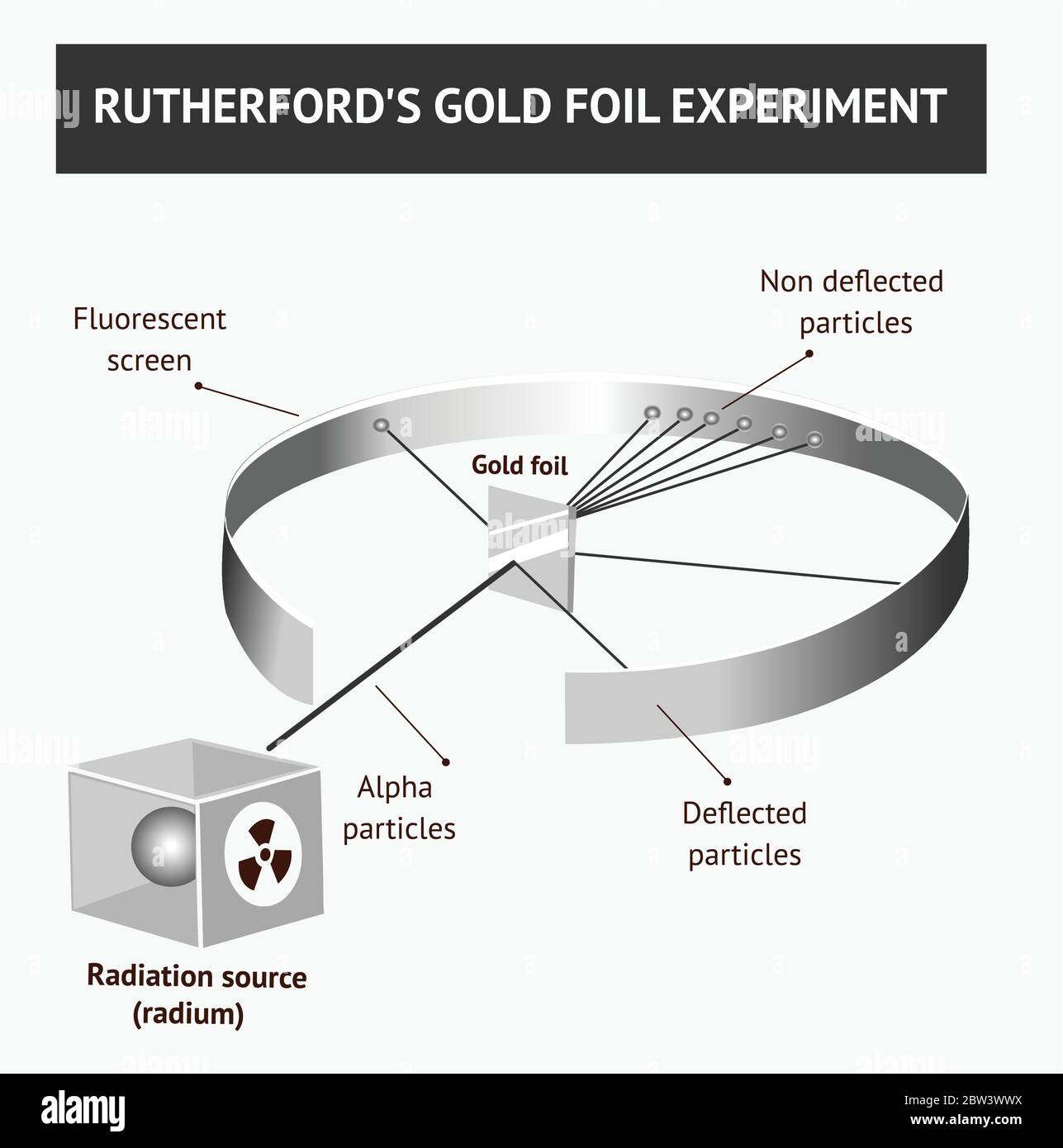
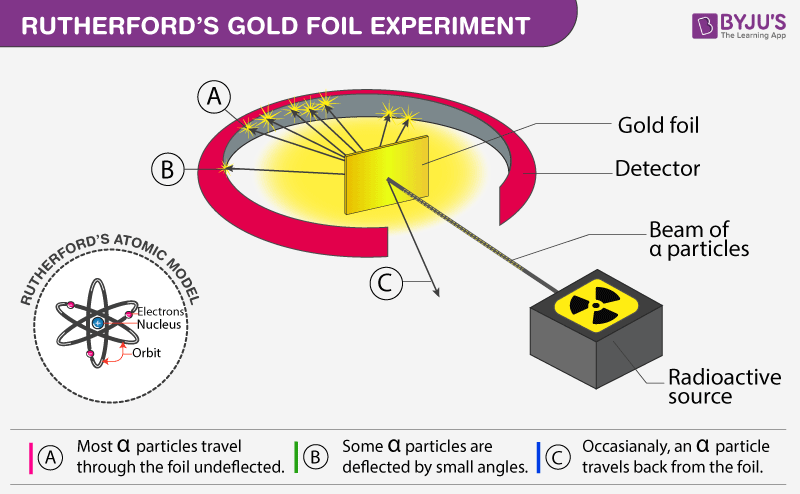
Rutherford's Alpha Scattering Experiment - GeeksforGeeks He conduct an experiment by bombarding alpha particles into a thin sheet of gold and then notices their interaction with the gold foil and trajectory or path followed by these particles. Atomic Theory, Model, and Bohr-Rutherford Diagrams- Google ... Atomic Model and Theory Lesson - The History of the Atom and Bohr-Rutherford Diagrams. This 27 slide Atomic Model lesson package examines the history of the atomic theory including the contributions of key persons as well as their influence on how the atom was perceived in their time including Democritus, Dalton (Ball Model), J.J. Thompson (Raisin Bun Model), Rutherford (Gold Foil Experiment ... Ernest Rutherford - Wikipedia Gold foil experiment. Top: Expected results: alpha particles passing through the plum pudding model of the atom undisturbed. Bottom: Observed results: a small portion of the particles were deflected, indicating a small, concentrated charge. Diagram is not to scale; in reality the nucleus is vastly smaller than the electron shell. Rutherford performed his most famous work after … explain the rutherford's gold foil experiment with the ... In Rutherford experiment, fast moving alpha particle were made to fall on thin gold foil. • This gold foil was about 1000 atoms thick. • Alpha Particles are doubly charged Helium ions. The following observations were made: Most of the fast moving alpha particle passed straight through the gold foil.
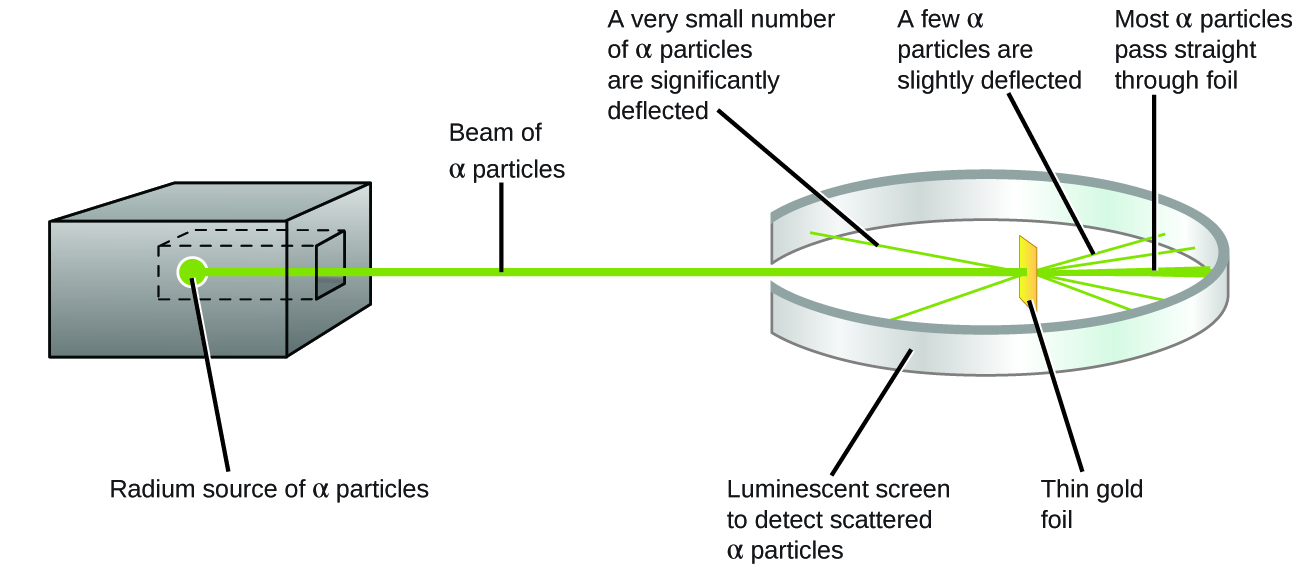
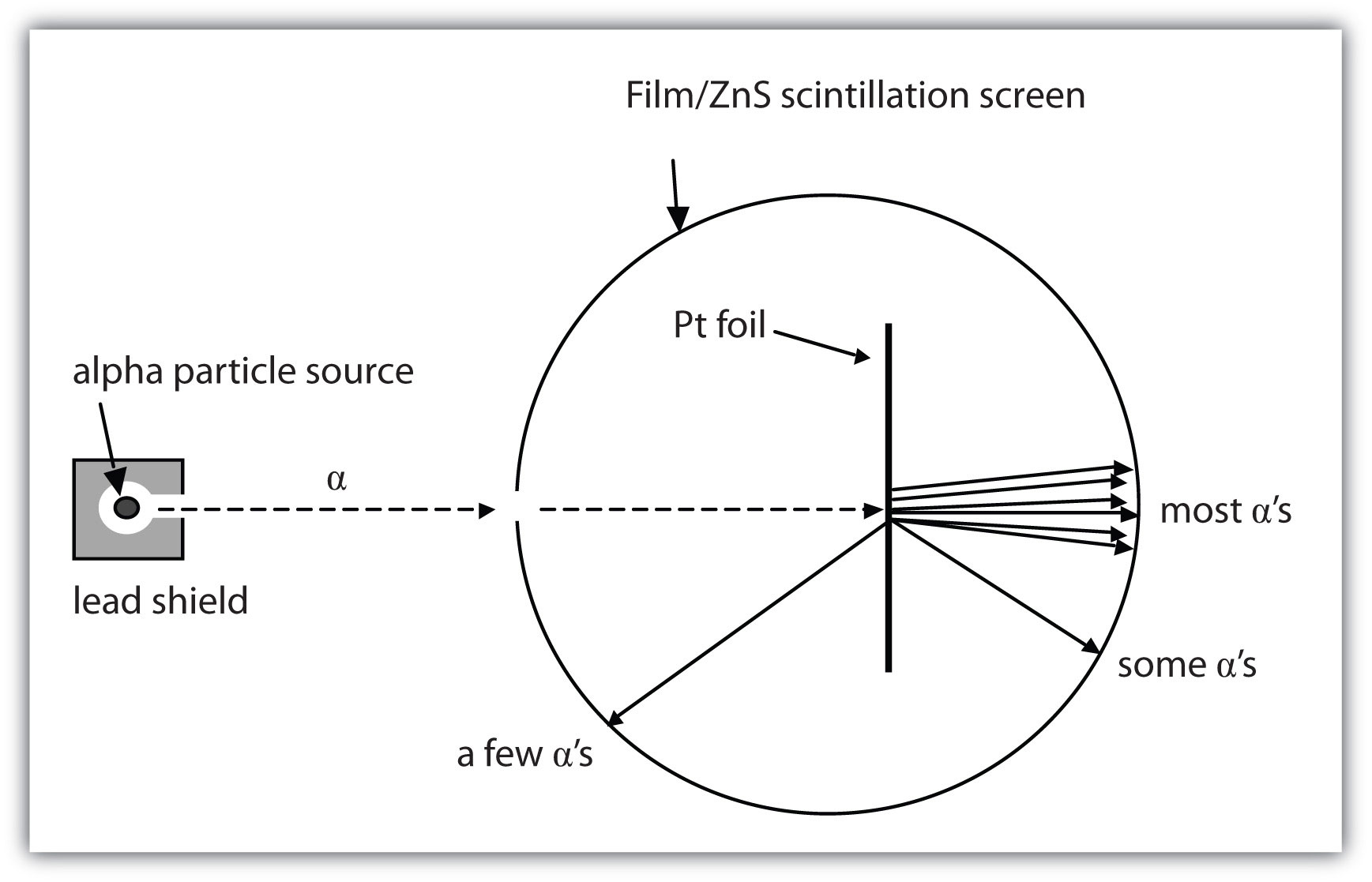
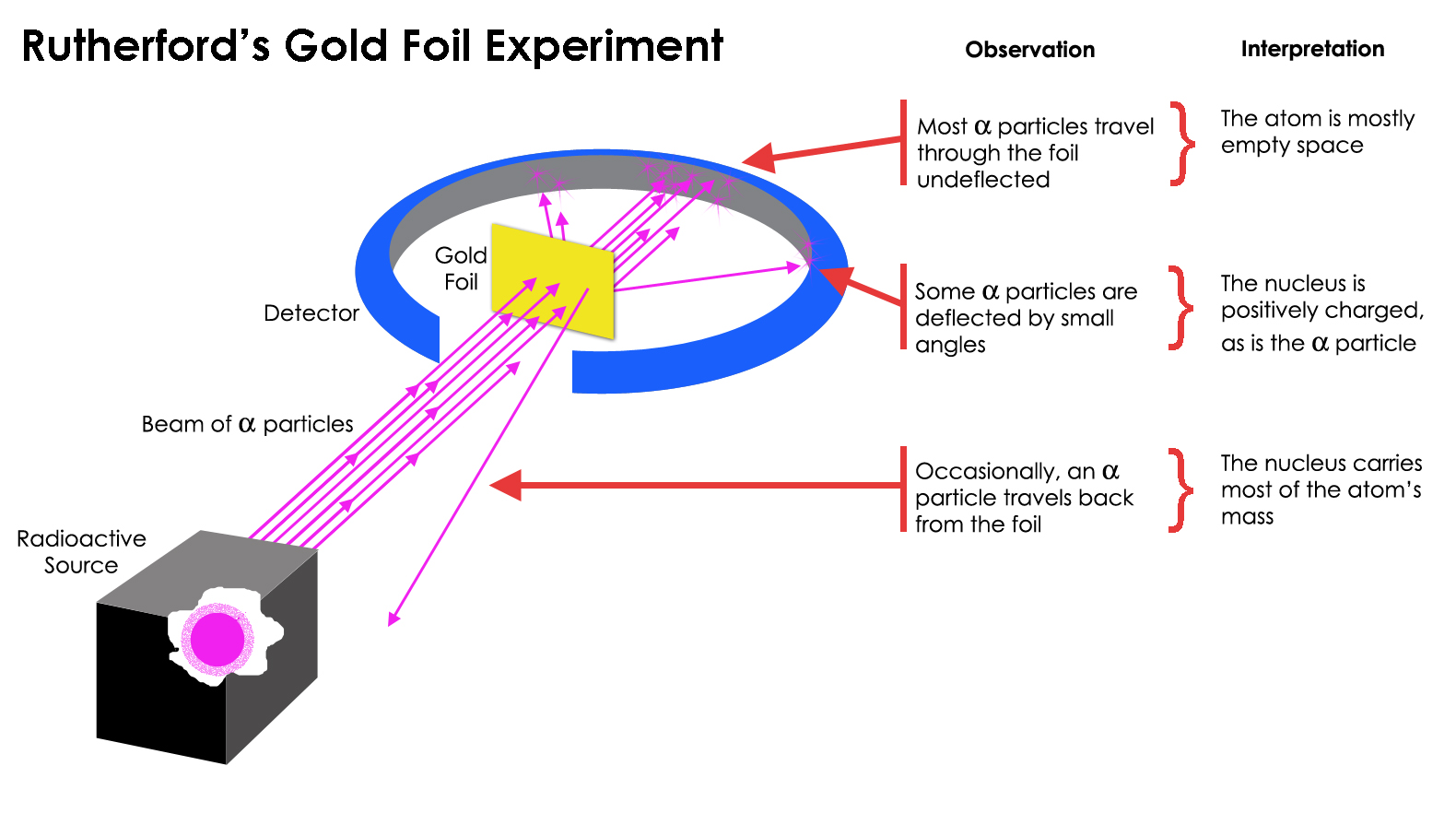
What are the conclusions of Gold foil Experiment ... Gold foil Experiment and conclusions: Helium Ions (α-particles) from a radioactive material (Radium) is passed through narrow slits to collimate them into straight-line path. Fast moving α-particles are made to hit a thin sheet (foil) of gold metal of about 100nm thickness. Rutherford's Experiments (M2Q2) - UW-Madison Chemistry 103 ... Geiger and Rutherford fired α particles at a piece of gold foil and detected where those particles went, as shown in this schematic diagram of their experiment. Most of the particles passed straight through the foil, but a few were deflected slightly and a very small number were significantly deflected. Rutherford Scattering: Experiment, Equation, Diagram Rutherford scattering diagram and description This is what Rutherford used in the experiment: A gold foil. For this experiment, Rutherford used a very thin gold foil. (Since gold is very malleable, it is possible to reduce its thickness to 0.00004cm.) A beam of alpha particles. Rutherford model - Wikipedia Experimental basis for the model. Rutherford overturned Thomson's model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny and heavy nucleus. Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. If Thomson was correct, the beam …
Rock Cycle – Definition, Steps, Importance, Diagram 02.11.2020 · 1) Formation of Igneous Rock – Melting, Cooling, and Crystallization. Magma, the molten rock present deep inside the earth, solidifies due to cooling and crystallizes to form a type of rock called igneous rocks.Cooling of igneous rocks can occur slowly beneath the surface of the earth or rapidly at its surface.
Gold Foil Experiment | Ernest Rutherford & Results - Video ... The Rutherford gold foil experiment worked by firing positively charged alpha particles through gold foil and observing where they ended up. To make their observations, Rutherford and his students...
Explain Rutherfords Experiment With the Help of A Diagram ... The gold foil was only 0.00004 centimeter thick. Most of the alpha particles went straight through the foil, but some were deflected by the foil and hit a spot on a screen placed off to one side. Geiger and Marsden found that about one in 20,000 alpha particles had been deflected 45o or more.
Explain Rutherford's alpha - ray scattering experiment ... 1. Most of the α -particles passed straight through the gold foil without any deviation. 2. Some of the α -particles were deflected by the foil by some angles. 3. Interestingly one out of every 12,000 alpha particles appeared to rebound. Conclusion of Rutherford's scattering experiment: 1.
Ernest Rutherford's Gold Foil Experiment: Physics Lab ... Ernest Rutherford's gold foil experiment helped scientists understand the charge of an atom. Follow along with Rutherford's experiment in this lab and understand the surprising results and ...
Rutherford's gold foil experiment (video) - Khan Academy Rutherford's gold foil experiment. This is the currently selected item. Bohr's model of hydrogen. Next lesson. Bohr's model of the hydrogen atom. Video transcript - [Voiceover] This is a quote by a physicist as a comment on one of his experimental results. He said, about his experiment, he said, "It was as if you fired a 15-inch shell "at a ...
Solved 5. By referring to the diagram below, describe the ... Detecting screen Gold foil Sit Alpha partik emitter ; Question: 5. By referring to the diagram below, describe the observations that Rutherford made during his gold foil experiment, and what inferences could be made from these observations. Detecting screen Gold foil Sit Alpha partik emitter
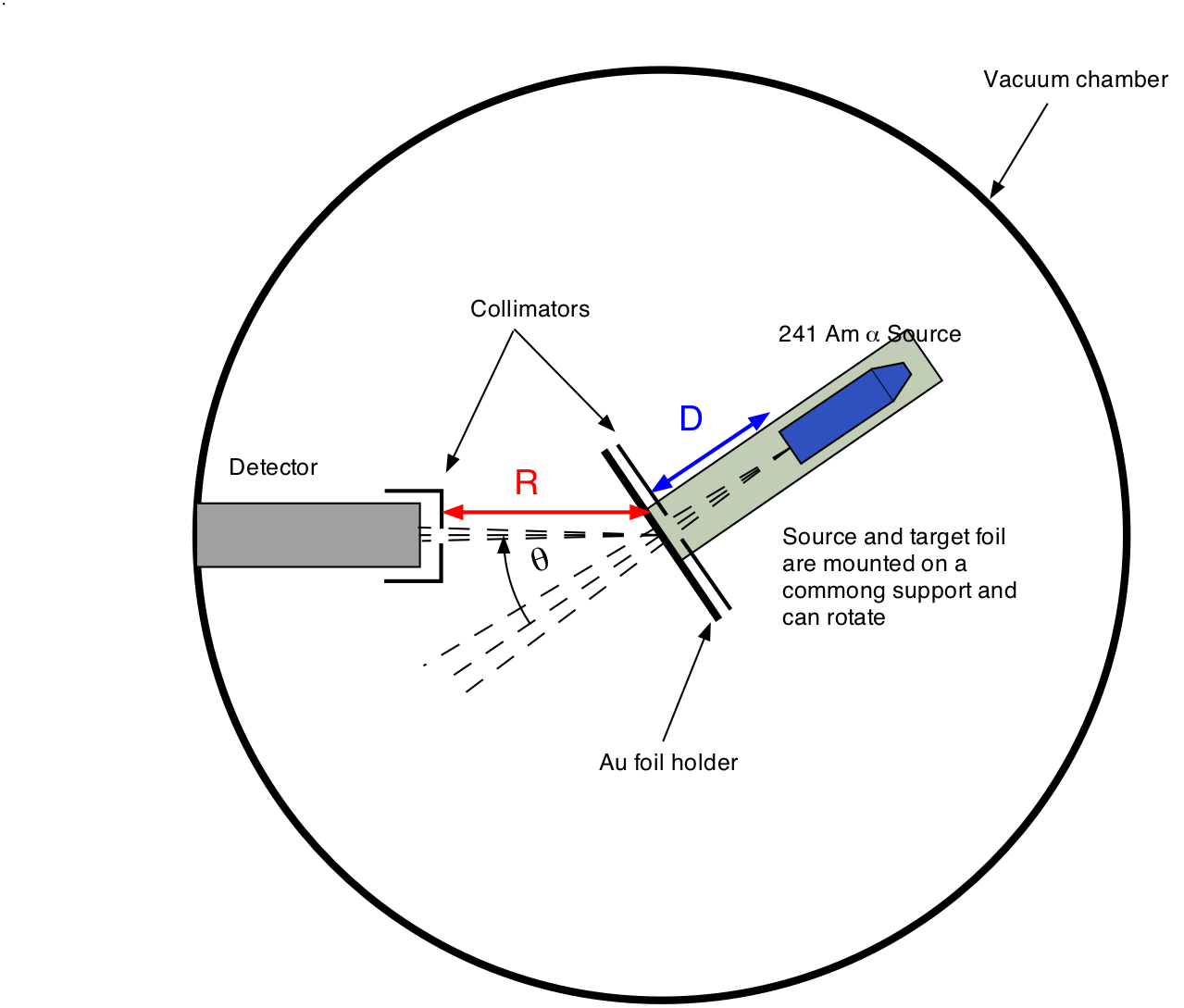
The Rutherford-Geiger-Marsden Experiment | PhysicsOpenLab 11.04.2017 · In the experiment, Rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). Surrounding the gold foil it was placed a zinc sulfide screen that would show a small flash of light when hit by a scattered alpha particle.
gold foil experiment Flashcards and Study Sets | Quizlet Gold foil experiment 1. Most particles passed through with n… 2. Some particles were deflected at acu… 3. Some particles were deflected back a… Symbol That meant the atom is mosty empty space That meant the nucleus was positively charged That meant the nucleus is dense Alpha particles 1. Most particles passed through with n…
What is Rutherford's Gold Foil Experiment - Pediaa.Com Rutherford's gold foil experiment (Rutherford's alpha particle scattering experiment) refers to an experiment carried out by Ernest Rutherford, Hans Geiger, and Ernest Marsden at the University of Manchester in the early 1900s. In the experiment, Rutherford and his two students studied how alpha particles fired at a thin piece of gold foil were deflected.
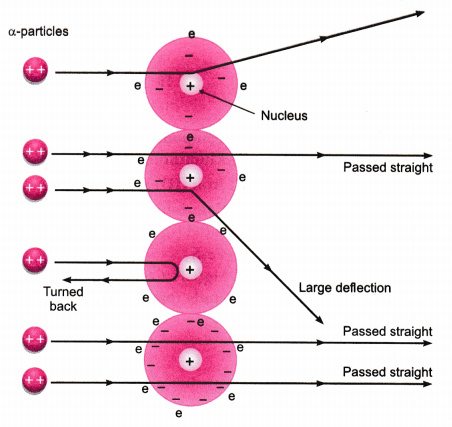
Rutherford's Gold Foil Experiment - Chemistry | Socratic When Rutherford shot α particles through gold foil, he found that most of the particles went through. Some scattered in various directions, and a few were even deflected back towards the source. He argued that the plum pudding model was incorrect.
Explain the gold foil experiment by Rutherford. What were ... Rutherford's Gold Foil Experiment: Rutherford took a very thin gold foil with 100 nm thickness. He bombarded the gold foil with alpha rays. It is important to note that alpha particles are positively charged.
Rutherford's Gold Foil Experiment - ChemistryGod All the experiments can be summarized using the illustration below. Rutherford's gold foil experiment When Rutherford along with his colleague shot alpha particles, the positively charged helium nuclei, on a very thin gold foil, unexpected scattering of the particles was observed.
PDF TEACHING TRANSPARENCY MASTER 11 Cathode Ray Experiments ... Which diagram depicts the plum pudding model of an atom? 3. Which diagram depicts Rutherford's actual results from his gold foil experiment? How did the actual results differ from the expected results? 4. What did Rutherford conclude from the results of his experiment? 5.
Rutherford and the nucleus - BBC Bitesize In 1905, Ernest Rutherford did an experiment to test the plum pudding model. His two students, Hans Geiger and Ernest Marsden, directed a beam of alpha particles at a very thin gold leaf suspended ...
Solved 14. In 1911, Ernest Rutherford tested Thomson's ... 14. In 1911, Ernest Rutherford tested Thomson's hypothesis by devising his "gold foil experiment i. With the help of a diagram discuss Ernest Rutherford's model. 11.5 marks) it. With the help of a diagram, explain the observations conclusion made by him he made from his experiment (4 marks) Question: 14.
Why did Rutherford select a gold foil in his alpha - ray ... For the scattering experiment, Rutherford wanted a metal sheet which could be as thin as possible. Gold is the most malleable of all known metals. It can easily be converted into very thin sheets. Hence, Rutherford selected a gold foil for his alpha-ray scattering experiment.
PDF The Gold foil experiment - WordPress.com The experiment was carried out in a vacuum, so deflection of the alpha particles must have been due to the gold foil. 1. Most alpha particles went straight through the gold foil, without any deflection. 2. Some alpha particles were slightly deflected by the gold foil. 3. A few alpha particles were bounced back from the gold foil.
diagram of Rutherford gold foil experiment and write the ... In this experiment, fast moving alpha (α)-particles were made to fall on a thin gold foil. He selected a gold foil because he wanted as thin a layer as possible. This gold foil was about 1000 atoms thick.α-particles are doubly-charged helium ions. Since they have a mass of 4µ, the fast-moving α-particles have a considerable amount of energy.
how to draw rutherford scattering experiment| how to draw ... Title: how to draw rutherford scattering experiment| how to draw diagram of rutherford gold foil experiment | rutherford alpha particle scattering experiment...
Exploring atoms: atom structure - Scootle See how scientists such as Ernest Rutherford have investigated the structure of atoms. Explore possible models. Fire charged particles at atoms and find which model best fits the results. This learning object is one in a series of six objects. Three of the objects are also packaged as a combined learning object.
Gold Foil Experiment- Video quiz. Diagram | Quizlet Diagrams Flashcards Mobile Help Sign up Help Center Honor Code Community Guidelines Students Teachers About Company Press Careers Advertise Privacy Terms Follow us Language DeutschEnglish (UK)English (USA)EspañolFrançais (FR)Français (QC/CA)Bahasa IndonesiaItalianoNederlandspolskiPortuguês (BR)РусскийTürkçeTiếng Việt한국어中文 (简体)中文 (繁體)日本語




















0 Response to "40 gold foil experiment diagram"
Post a Comment