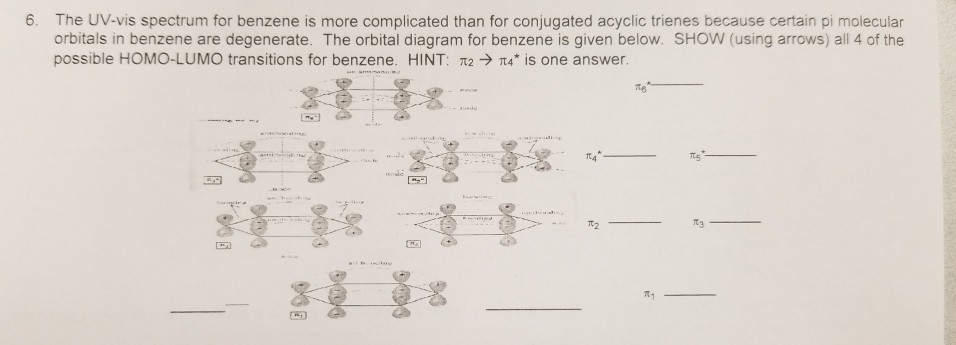
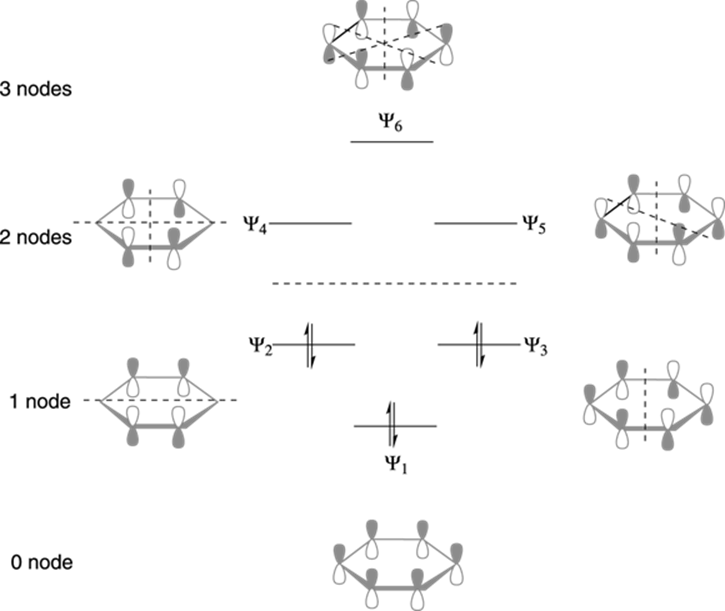
40 benzene molecular orbital diagram
en.wikipedia.org › wiki › Sigma_bondSigma bond - Wikipedia The corresponding antibonding, or σ* orbital, is defined by the presence of one nodal plane between the two bonded atoms. Sigma bonds are the strongest type of covalent bonds due to the direct overlap of orbitals, and the electrons in these bonds are sometimes referred to as sigma electrons. The symbol σ is the Greek letter sigma. socratic.org › questions › what-are-nonbondingWhat are nonbonding molecular orbitals? + Example Apr 13, 2014 · A non-bonding orbital (NBMO) is a molecular orbital for which the addition or removal of an electron does not change the energy of the molecule. Molecular orbitals come from the linear combination of atomic orbitals. In a simple diatomic molecule such as HF, F has more electrons than H. The s orbital of H can overlap with the 2p_z orbital of fluorine to form a bonding σ and an antibonding σ ...
› Calculate-Bond-Order-in-Chemistry3 Ways to Calculate Bond Order in Chemistry - wikiHow Jan 18, 2022 · In molecular orbital theory, bond order is also defined as half of the difference between the number of bonding and antibonding electrons. For a straightforward answer: use this formula: Bond order = [(Number of electrons in bonding molecules) - (Number of electrons in antibonding molecules)]/2 .

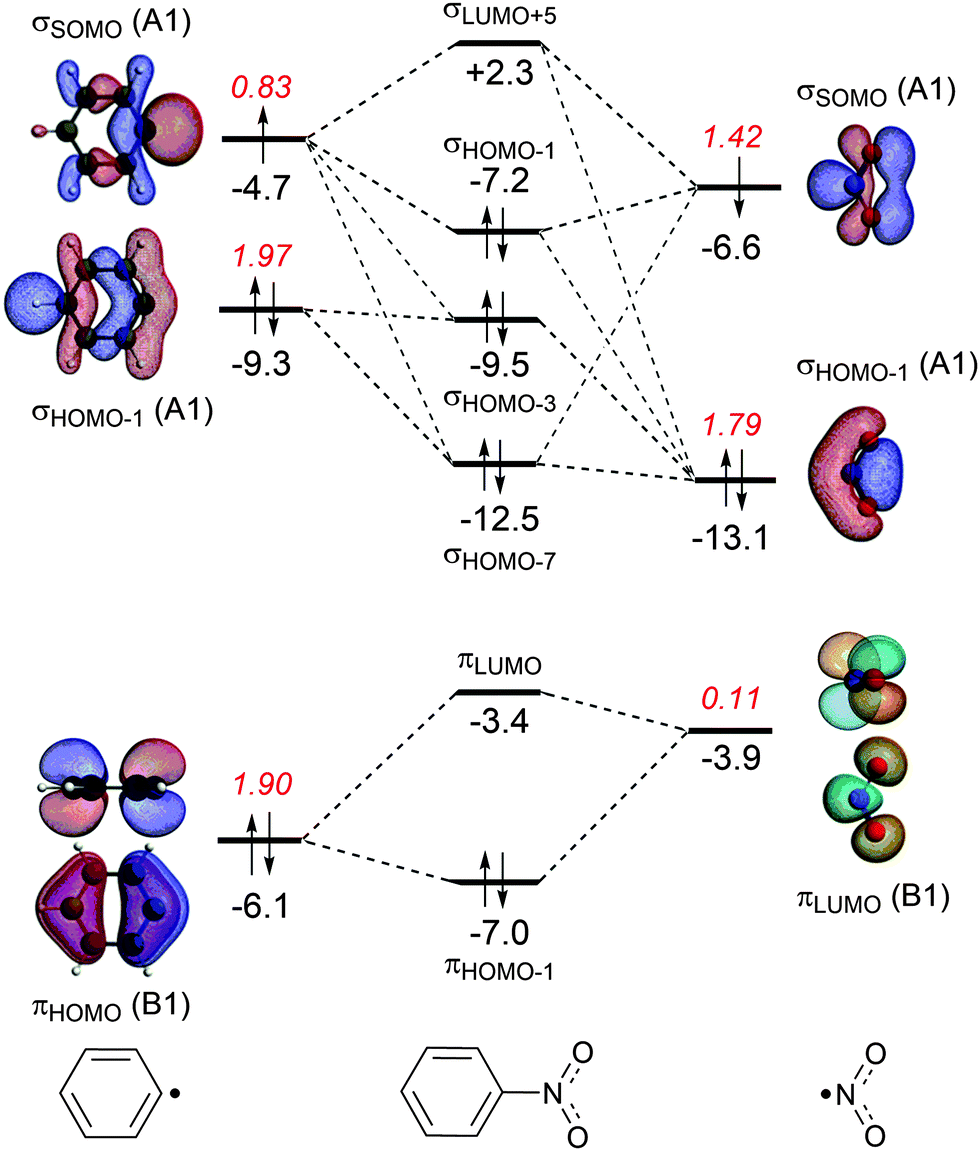
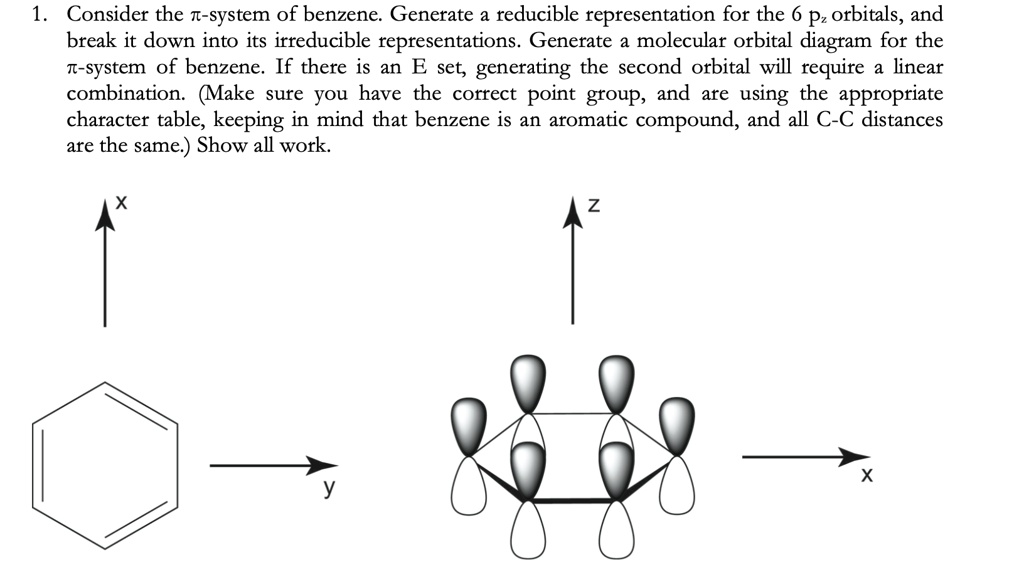
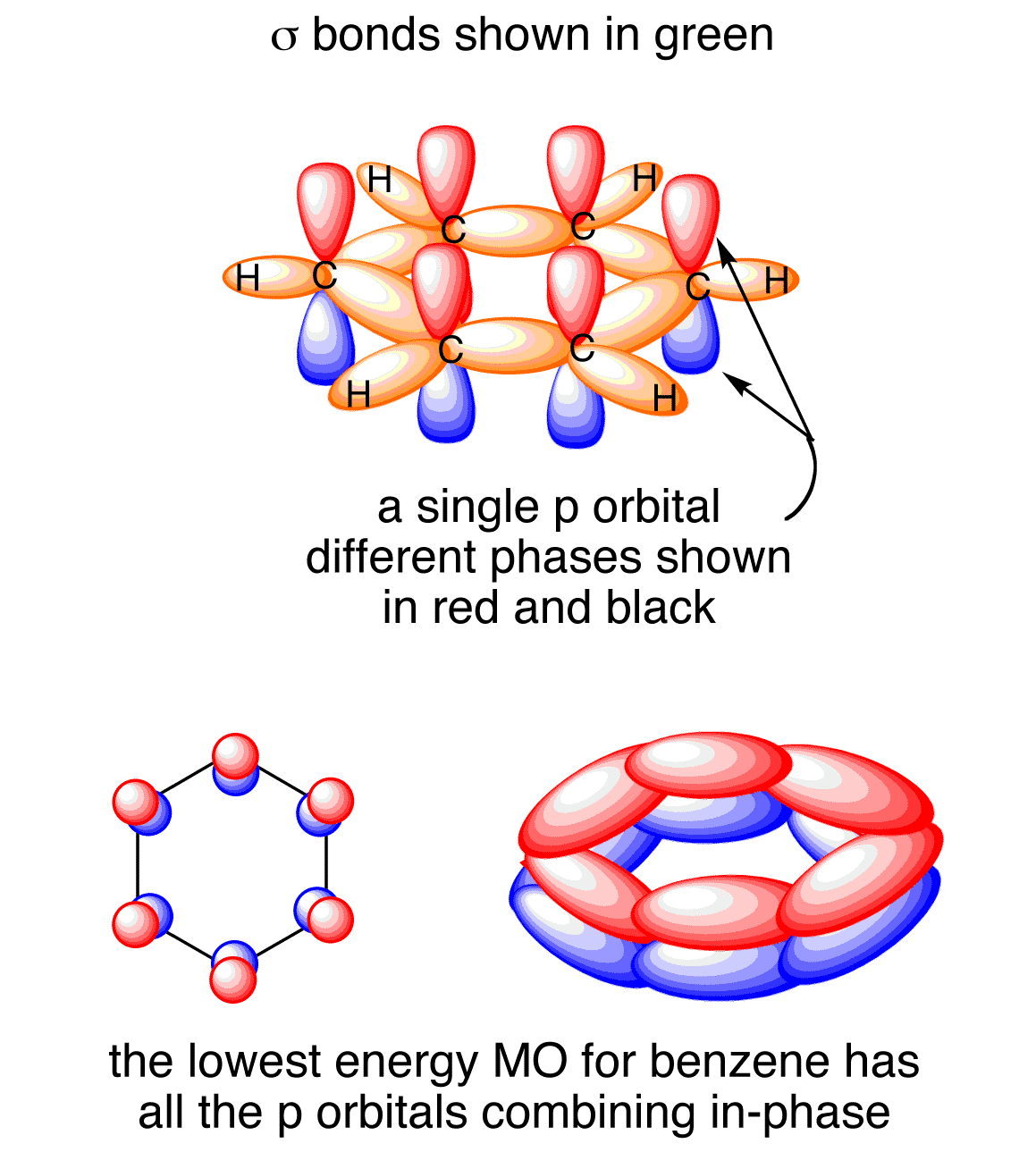
Benzene molecular orbital diagram
Molecular Orbitals of The Allyl Cation, Allyl Radical, and ... 16.02.2017 · In the last post, we showed how to build a molecular orbital (MO) diagram for a typical C-C pi bond. We saw that: ... For example the lowest energy MO of benzene has zero nodes, but the next-highest energy level of benzene is “doubly degenerate” meaning that there are two ways to draw a single nodal plane. More here. We’ll cover this in a separate post. [An … en.wikipedia.org › wiki › Covalent_bondCovalent bond - Wikipedia A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. Non-bonding orbital - Wikipedia A non-bonding orbital, also known as non-bonding molecular orbital (NBMO), is a molecular orbital whose occupation by electrons neither increases nor decreases the bond order between the involved atoms.Non-bonding orbitals are often designated by the letter n in molecular orbital diagrams and electron transition notations. Non-bonding orbitals are the equivalent in …
Benzene molecular orbital diagram. www2.chemistry.msu.edu › faculty › reuschPhotochemistry - Michigan State University In the sp 2-oxygen model these occupy very similar (degenerate) orbitals, but in the sp-oxygen model one pair is in a relatively low energy sp-orbital and the other in a higher energy p-orbital. Molecular orbital calculations clearly show that the latter model is a better representation than the former. Resonance (chemistry) - Wikipedia In benzene the two cyclohexatriene Kekulé structures, first proposed by Kekulé, are taken together as contributing structures to represent the total structure.In the hybrid structure on the right, the dashed hexagon replaces three double bonds, and represents six electrons in a set of three molecular orbitals of π symmetry, with a nodal plane in the plane of the molecule. Non-bonding orbital - Wikipedia A non-bonding orbital, also known as non-bonding molecular orbital (NBMO), is a molecular orbital whose occupation by electrons neither increases nor decreases the bond order between the involved atoms.Non-bonding orbitals are often designated by the letter n in molecular orbital diagrams and electron transition notations. Non-bonding orbitals are the equivalent in … en.wikipedia.org › wiki › Covalent_bondCovalent bond - Wikipedia A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
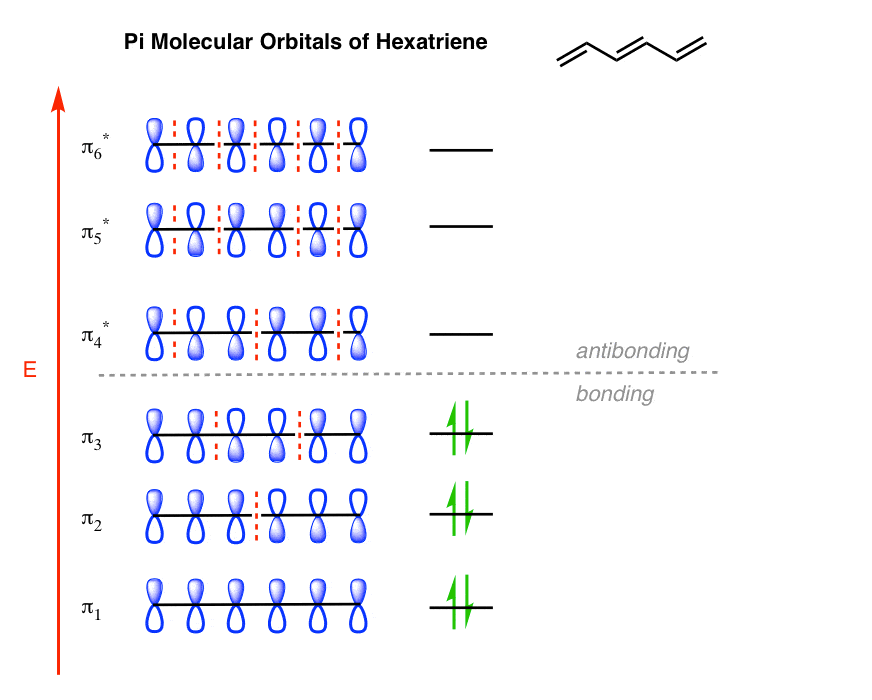
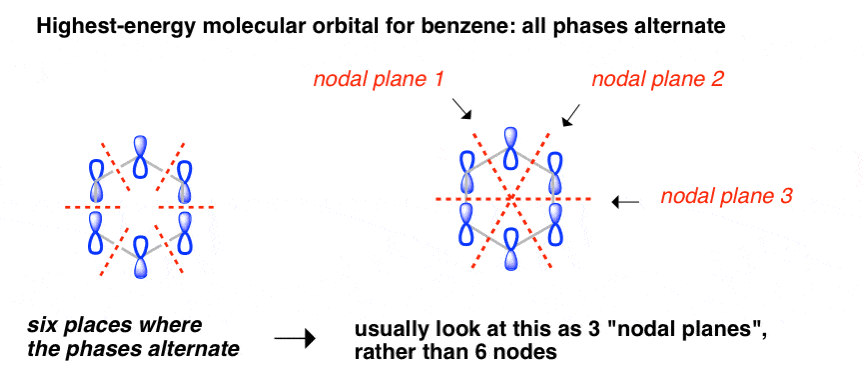
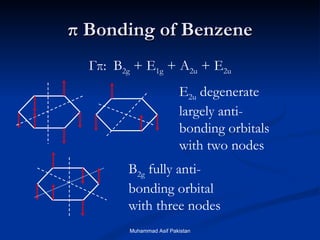
Molecular Orbitals of The Allyl Cation, Allyl Radical, and ... 16.02.2017 · In the last post, we showed how to build a molecular orbital (MO) diagram for a typical C-C pi bond. We saw that: ... For example the lowest energy MO of benzene has zero nodes, but the next-highest energy level of benzene is “doubly degenerate” meaning that there are two ways to draw a single nodal plane. More here. We’ll cover this in a separate post. [An …





















0 Response to "40 benzene molecular orbital diagram"
Post a Comment