39 energy diagram endothermic and exothermic reaction
🔥🔥Exothermic Reactions🔥🔥 - jeopardylabs.com What is an exothermic reaction?, If the energy change on an energy level diagram is negative, what does this tell you about the reaction?, What does the law of conservation state?, If a reaction releases more energy when new bonds are made than the amount of energy absorbed to break bonds in the reactants. What type of reaction is this? Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
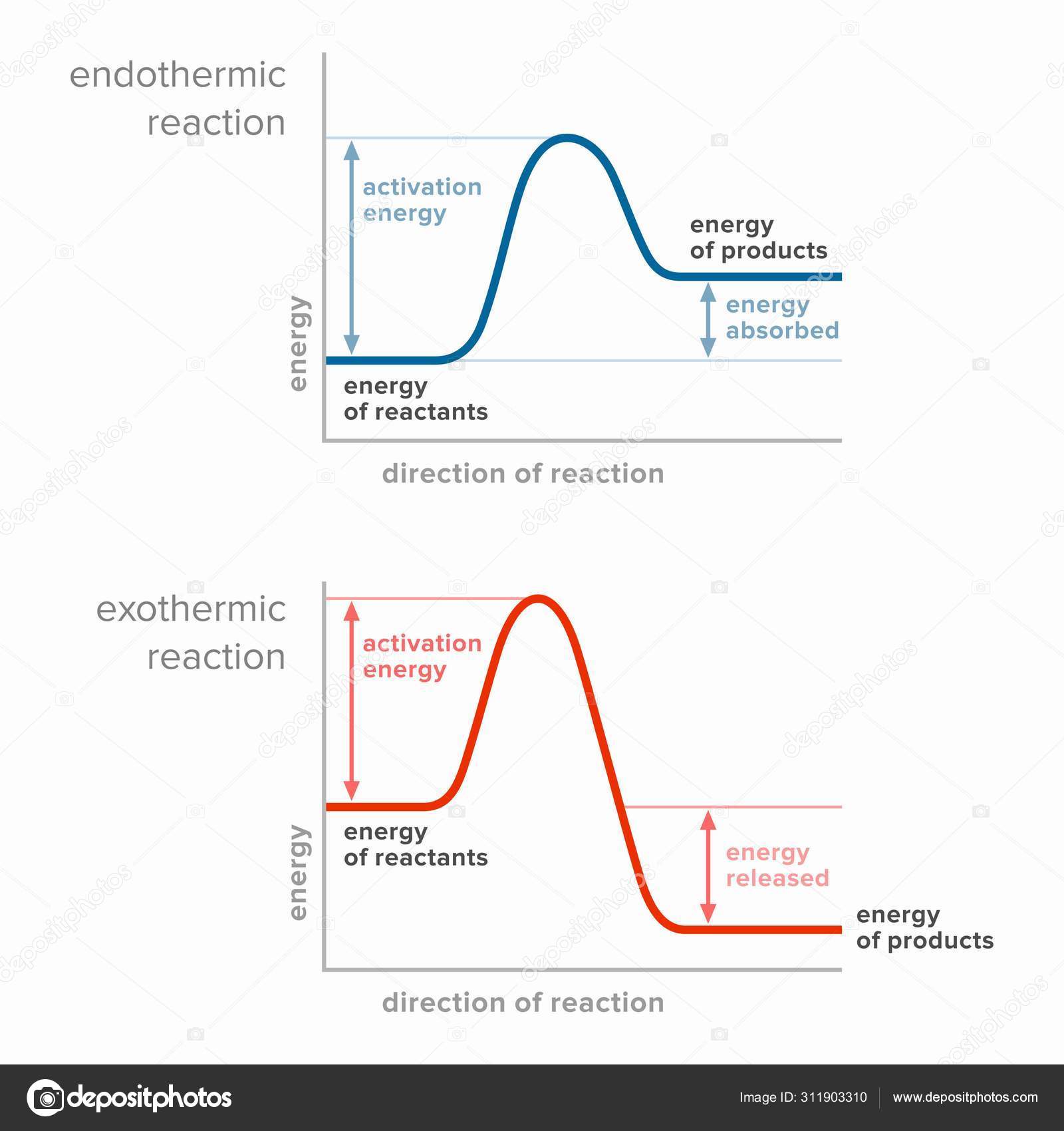
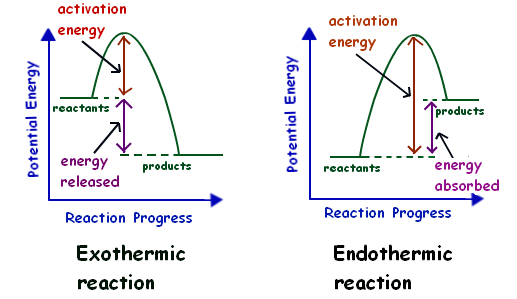
Endothermic vs. exothermic reactions (article) | Khan Academy Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants.
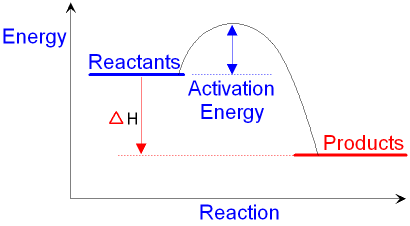
Energy diagram endothermic and exothermic reaction
PDF Benifits Of Exothermic And Endothermic Reaction May 1st, 2018 - What Is The Difference Between Endothermic And Exothermic Reactions Energy Should Be Given To The System In Endothermic Reactions But Energy Is Released''BBC GCSE Bitesize Exothermic Reactions April 28th, 2018 - A Secondary School Revision Resource For AQA GCSE Additional Science About Chemical Reaction Coordinate Diagram Endothermic Vs Exothermic A reaction will be exothermic if the energy of the products is less than the energy of the Below is a reaction coordinate diagram for an endothermic reaction. A general Reaction Coordinate Diagram relating the energy of a system to leading to an exothermic reaction (∆H 0). Endothermic Reaction. Difference Between Endothermic and Exothermic Reactions ... Similar is the case with the endothermic and exothermic reactions in Chemistry. These release energy in the form of sound, light, cold or heat. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. On the other hand, an exothermic reaction releases energy into the surrounding of the system.
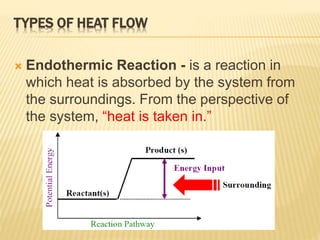
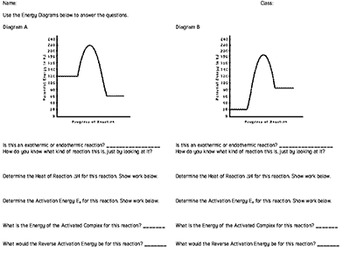
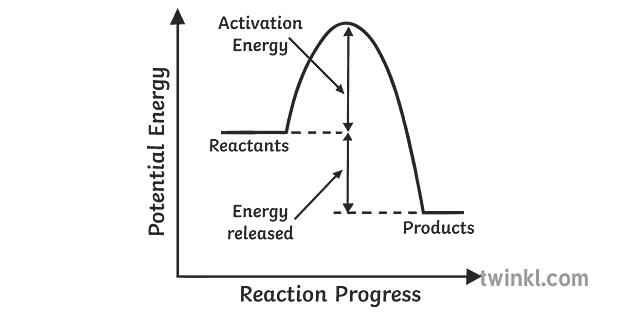
Energy diagram endothermic and exothermic reaction. Exothermic and endothermic reactions - Energy changes in ... Exothermic and endothermic reactions When a chemical reaction occurs, energy is transferred to or from the surroundings. There is usually a temperature change. For example, when a bonfire burns it... Energy Diagrams of Reactions | Fiveable Energy Diagrams Physical or chemical processes can be described through energy diagrams. As mentioned before, reactions can be categorized as endothermic or exothermic processes. The energy diagrams below show what should be known for the test. Image Courtesy of Pinterest Before looking at the specifics of each, you should be aware of a few terms: Endothermic and Exothermic Activity.docx - Endothermic and ... Conclusion Statement The potential energy diagram is used to identify if the reaction is endothermic or exothermic. We determine this by finding if the diagram has a higher potential energy than the reactants. If it is higher, we know the reaction is endothermic because this explains why the reaction absorbs heat from its surroundings. If the diagram shows the reactants having a higher ... PPTX Energy and Chemical Reactions - Boyertown Area School ... Endothermic and Exothermic reactions. Step 1: Energy must be SUPPLIED to break chemical bonds of reactants: Step 2: Energy is RELEASED when new chemical bonds are made in the products: A reaction is . EXOTHERMIC . if more energy is . RELEASED . than. SUPPLIED. If more energy is . SUPPLIED. than is . RELEASED. then the reaction is . ENDOTHERMIC ...
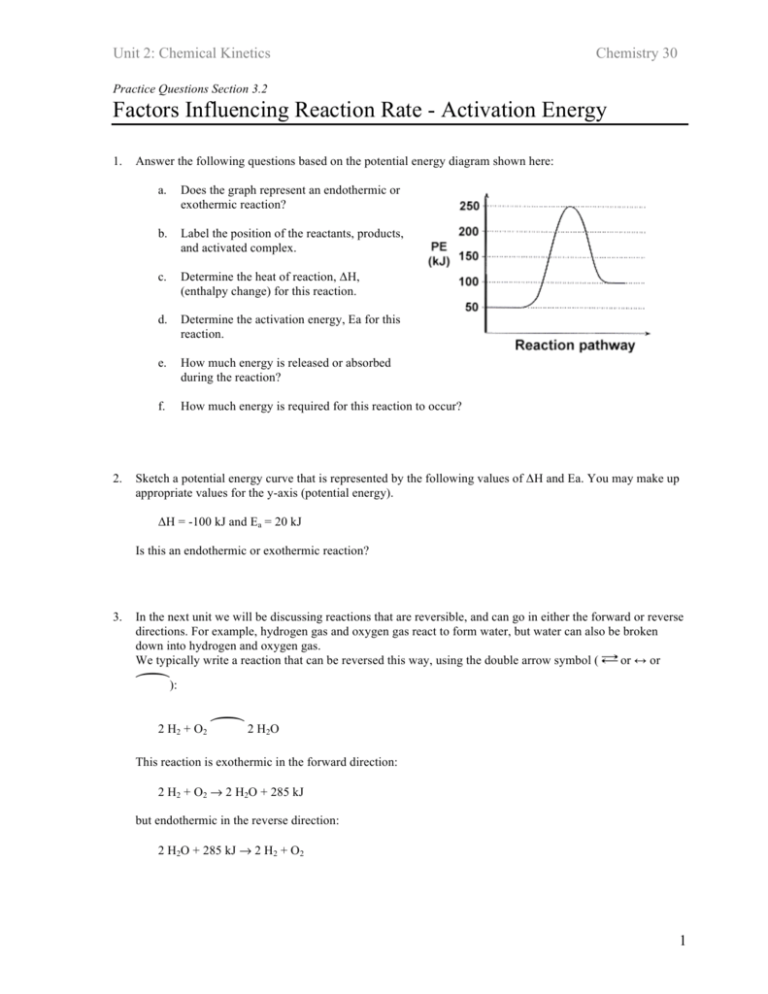
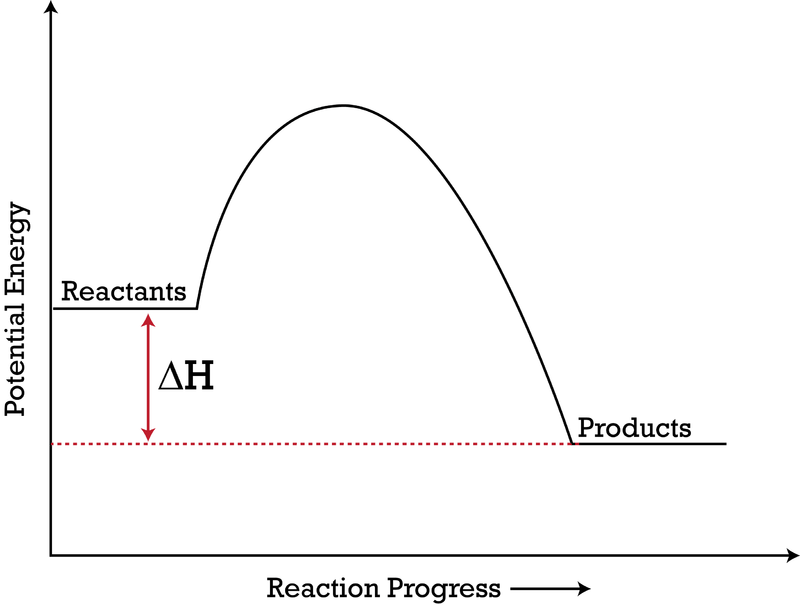
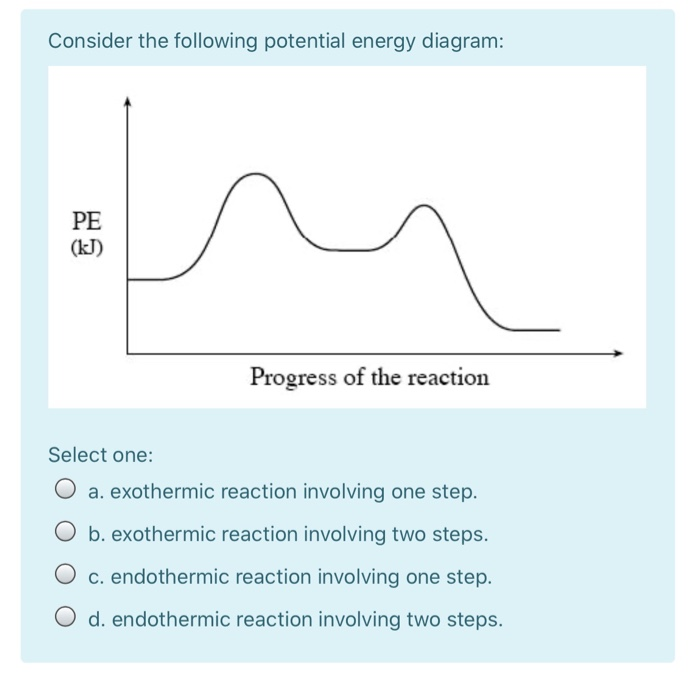
What are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant. How do you know if a diagram is endothermic or exothermic ... How do you know if a diagram is endothermic or exothermic? In the energy level diagram, the enthalpies of the products are lower than that of the reactants. Hence, the enthalpy change is negative (ΔH<0). By examining this enthalpy change, one can tell whether a reaction is endothermic (ΔH>0) or exothermic (ΔH<0). 14 Sketch a potential energy diagram for an exothermic ... 14. Sketch a potential energy diagram for an exothermic reaction and for an endothermic reaction. Label the axes, reactants, products, heat of reaction, activation energy, and transition state on each diagram. 15. The following data were obtained for the decomposition of dinitrogen pentoxide at 45 C. N 2 O 5 (mol/L) Time (min) 0.316 0 0.274 39 0.238 80 0.190 140 0.146 210 a) Plot a graph of ... Draw an energy diagram for a three-step reaction. First ... Draw an energy diagram for a three-step reaction. First step is exothermic, second and third steps are endothermic. First step is the slowest; the last step is the fastest. The overall process is endothermic. Indicate on the energy diagram all the Ea's, intermediates, reactants, products, transition states and all the-H's (Each feature ...
Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ... Examples of Endothermic Reactions - ThoughtCo PDF 5.1 - Exothermic and Endothermic Reactions Exothermic Reaction- A reaction that causes the temperature of the surroundings to increase. Energy is lost, or released, in the reaction, as the enthalpy of the products is less than the enthalpy of the reactants. Endothermic Reaction- A reaction that causes the temperature of the surroundings to decrease. Real Life Examples of Exothermic Reactions for IIT JEE - Vedantu
🔥🔥Exothermic Reactions🔥🔥 Jeopardy Template What is an exothermic reaction?, If the energy change on an energy level diagram is negative, what does this tell you about the reaction?, What does the law of conservation state?, If a reaction releases more energy when new bonds are made than the amount of energy absorbed to break bonds in the reactants. What type of reaction is this?
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
Endothermic Reactions - Definition and Examples - Science Notes
EXOTHERMIC & ENDOTHERMIC REACTIONS: ENERGY ... ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages
PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... c) Draw a labeled enthalpy level diagram for an exothermic and endothermic reaction showing the activation energy, Ea and enthalpy change. [4] 9. (M05/S/2) In a neutralization reaction 50 cm 3 of a 0.50 moldm-3 solution of sodium hydroxide is mixed rapidly in a glass beaker with 50 cm 3 of a 0.050 moldm-3 solution of sulfuric acid.
CH103 – Chapter 7: Chemical Reactions in Biological Systems
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. This means that there was more energy in reactants than in the products.
what compound directly provides energy - Lisbdnet.com
Representing endothermic and exothermic processes using ... Representing endothermic and exothermic processes using energy diagrams AP.Chem: ENE‑2.B (LO) , ENE‑2.B.1 (EK) Transcript A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state.
QUESTIONS: 1. Use The Reaction Energy Diagram Below To ... QUESTIONS: 1. Use The Reaction Energy Diagram Below To Answer The Following Question(S). (10 Points) Energy Reaction Progress The Reaction Depicted In This Reaction Energy Diagram Can Best Be Described As A) A Slow Exothermic Reaction B) A Fast Exothermic Reaction C) A Slow Endothermic Reaction D) A Fast Endothermic Reaction (I) (Ii) The Transition State Is
Is melting endothermic or exothermic? - All Famous Faqs Melting is an endothermic reaction in which the total amount of heat in the substance, also known as the enthalpy, increases. Why is ice melting an endothermic reaction? In order to melt the ice cube, heat is required, so the process is endothermic. Endothermic reactionIn an endothermic reaction, the products are higher in energy than the ...
Exothermic reaction - Wikipedia
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
How does the energy level diagram show this reaction is ... Energy profile diagrams for endothermic and exothermic reactions Every chemical substance has a certain amount of chemical energy. This energy is given the symbol H and is different for different substances. It is difficult to measure the absolute energy of a substance but the change in energy during chemical reactions can be easily measured.
Exothermic Reactions - Definition and Examples - Science Notes
Bio 2.2 Biochemical Reactions Flashcards | Quizlet
Exothermic and Endothermic Reactions - Energy Level Diagram Exothermic and Endothermic Reactions - Energy Level DiagramForm 5 Chemistry Chapter 4 ThermochemistryThis video is created by ...
53 1 point Does this energy diagram represent an | Chegg.com Transcribed image text: 53 1 point Does this energy diagram represent an endothermic or an exothermic reaction? How can you tell? D A B с TERRI-HR Paragraph BIV E 12pt A. I EX A TIR 1 point Click on the letter that corresponds to the activation energy on this energy diagram 0 А B 55 TO Click on the letter that corresponds to the change in Gies Free Energy on the energy diagram D B 56 Click ...
Difference Between Endothermic and Exothermic Reactions ... Similar is the case with the endothermic and exothermic reactions in Chemistry. These release energy in the form of sound, light, cold or heat. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. On the other hand, an exothermic reaction releases energy into the surrounding of the system.
Reaction Coordinate Diagram Endothermic Vs Exothermic A reaction will be exothermic if the energy of the products is less than the energy of the Below is a reaction coordinate diagram for an endothermic reaction. A general Reaction Coordinate Diagram relating the energy of a system to leading to an exothermic reaction (∆H 0). Endothermic Reaction.
PDF Benifits Of Exothermic And Endothermic Reaction May 1st, 2018 - What Is The Difference Between Endothermic And Exothermic Reactions Energy Should Be Given To The System In Endothermic Reactions But Energy Is Released''BBC GCSE Bitesize Exothermic Reactions April 28th, 2018 - A Secondary School Revision Resource For AQA GCSE Additional Science About Chemical


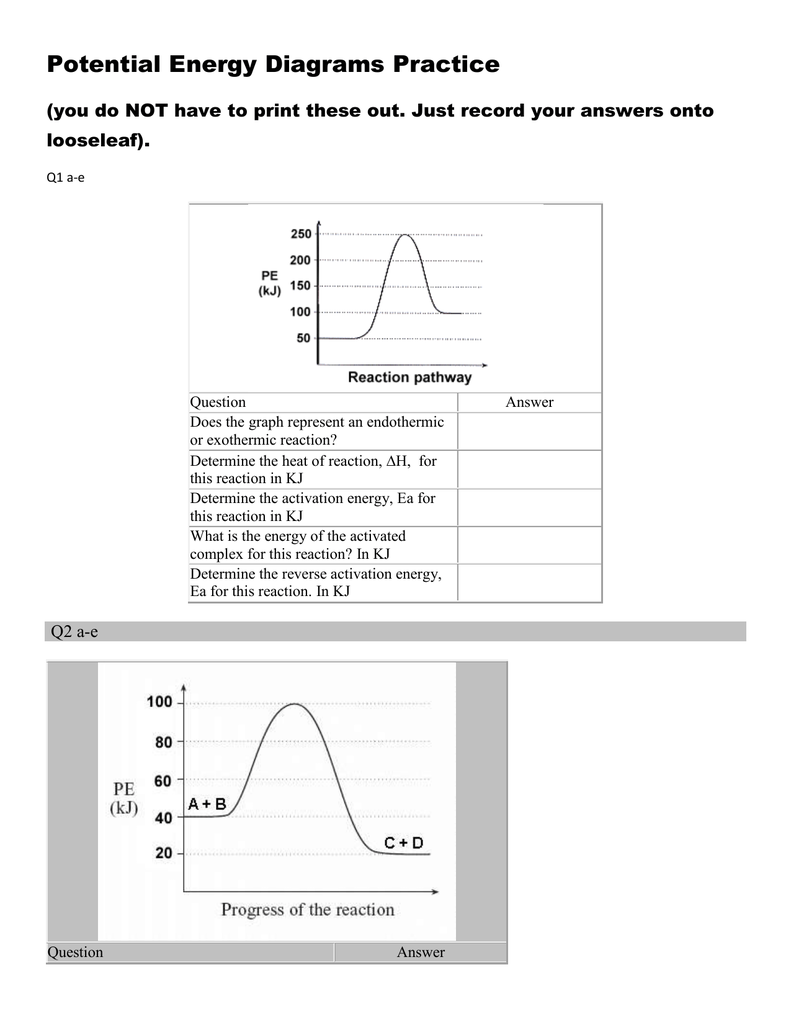












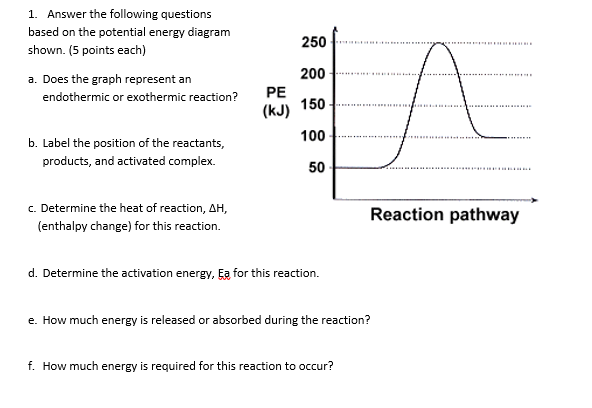
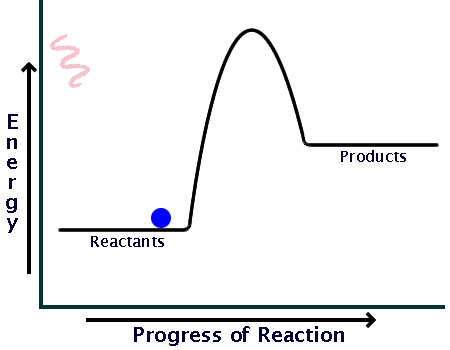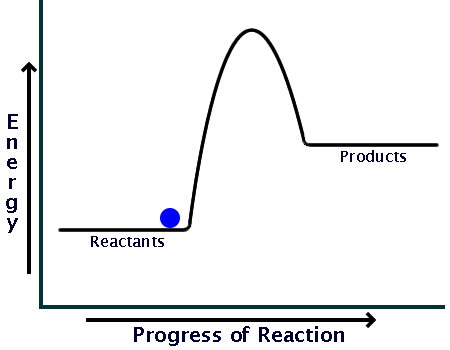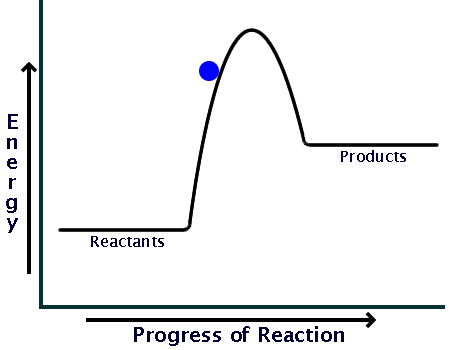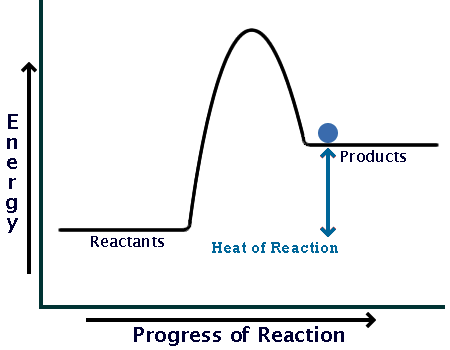






0 Response to "39 energy diagram endothermic and exothermic reaction"
Post a Comment