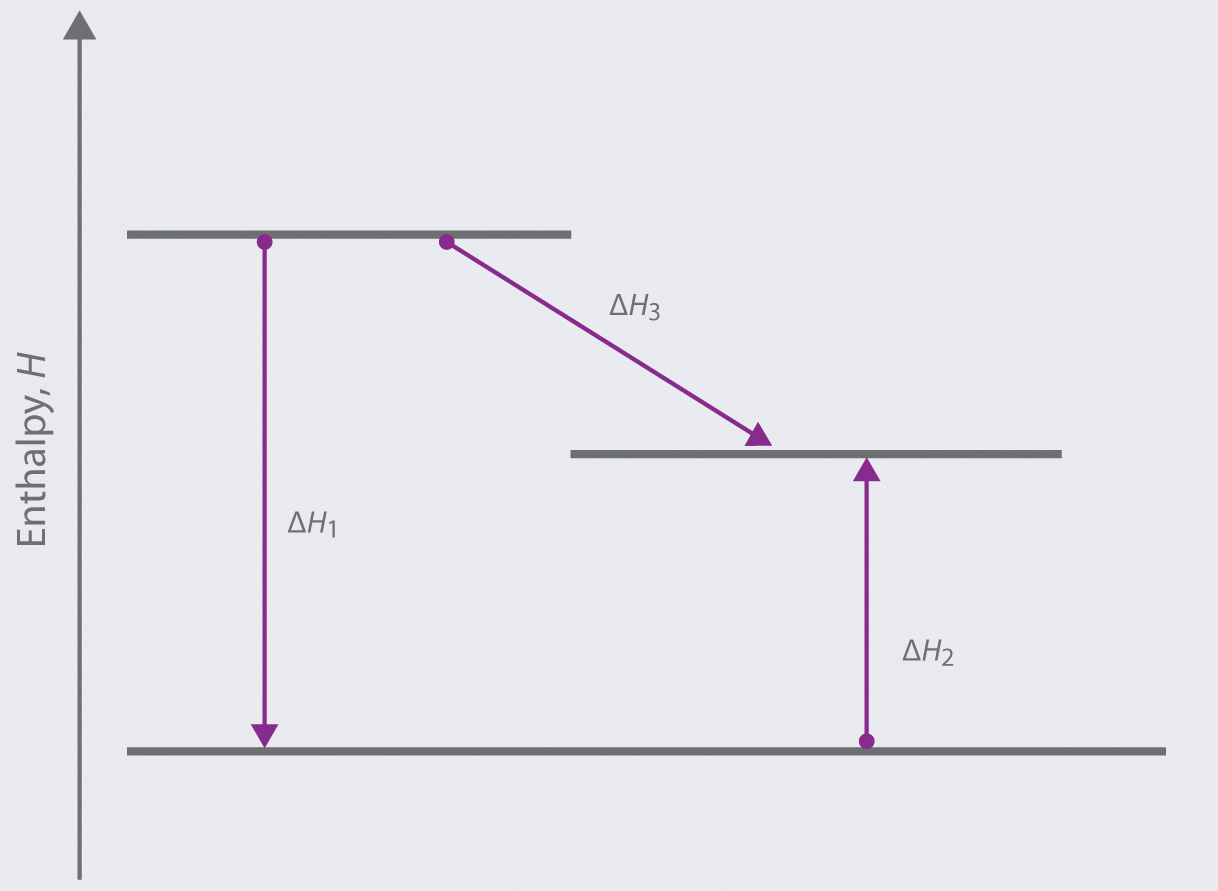
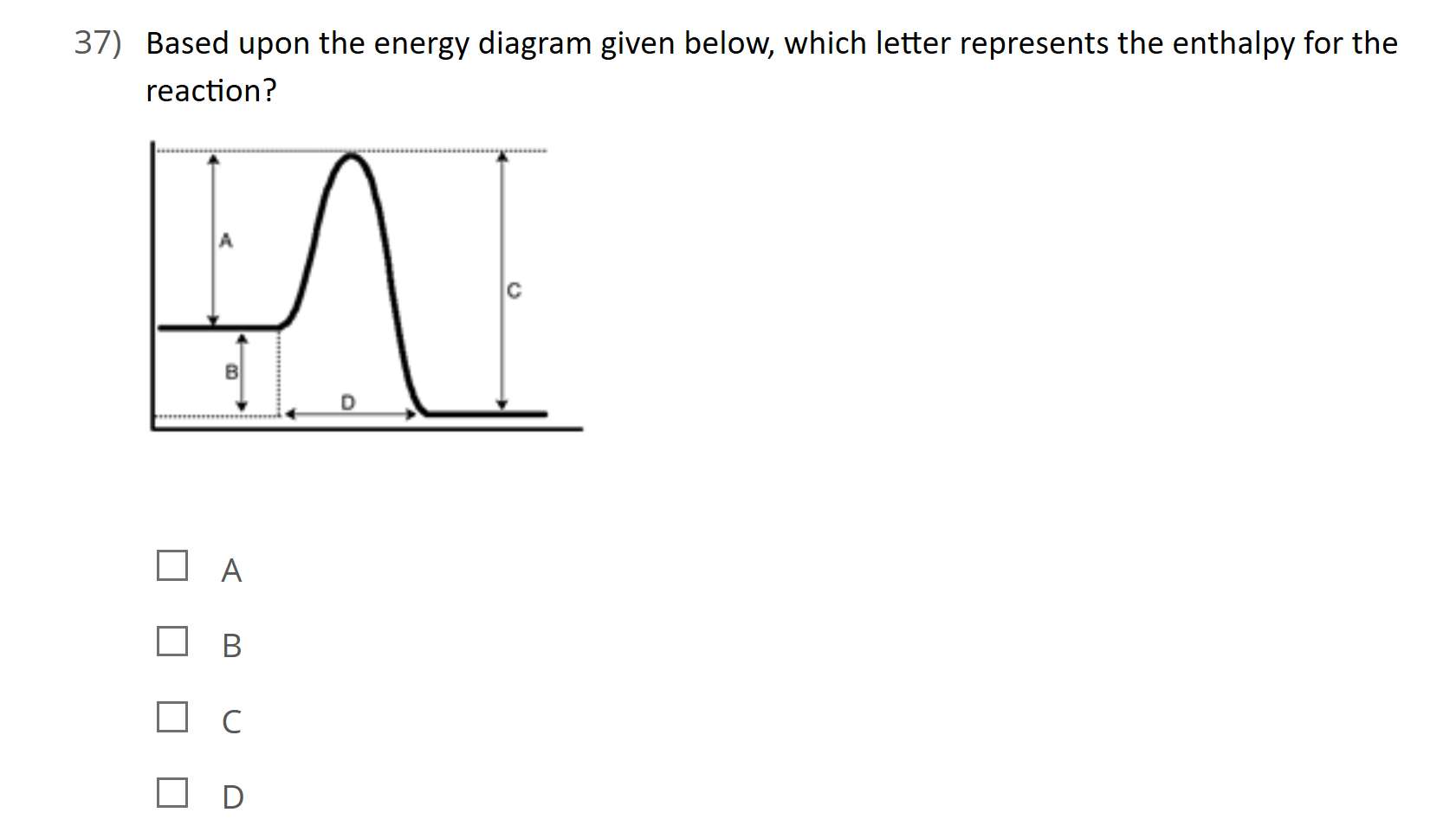
37 this diagram would represent the enthalpy change in which of the following
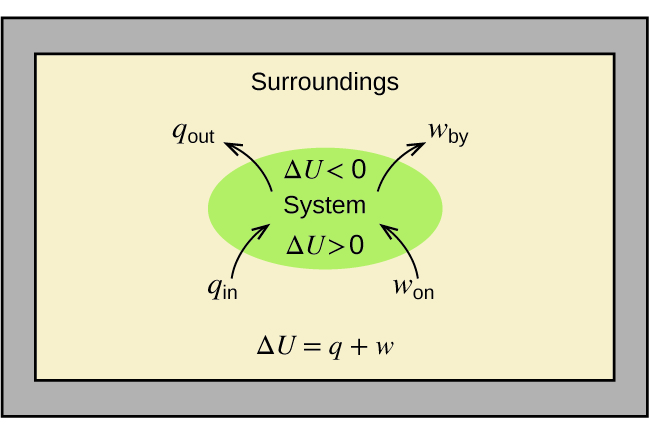
1 Chapter 5 assignment.pdf - Name Chapter 5 Communication ... View 1 Chapter 5 assignment.pdf from CHEMISTRY CHEM 3FF3 at McMaster University. Name Chapter 5 Communication Assignment [Total marks /50] K/U = /10 1. Create a simple enthalpy diagram to represent How to Draw & Label Enthalpy Diagrams - Video & Lesson ... An enthalpy diagram plots information about a chemical reaction such as the starting energy level, how much energy needs to be added to activate the reaction, and the ending energy. An enthalpy...
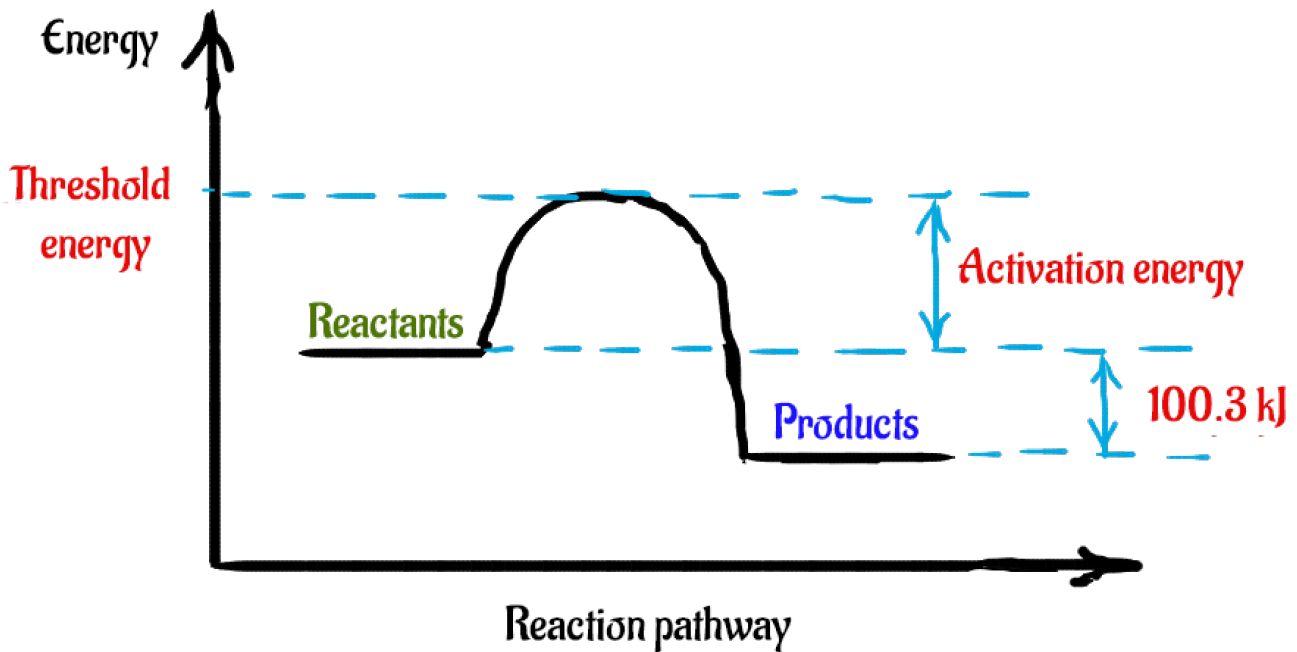
PDF Representing a Reaction with a Potential Energy Diagram Determine the activation energy and the enthalpy change for the reaction. b. Draw a labelled potential energy diagram for the reaction, indicating the relative energies of the reactants, products, and activated complex. What Is Required? You need to determine the activation energy, E a, and the enthalpy change, ΔH r, for the reaction.
This diagram would represent the enthalpy change in which of the following
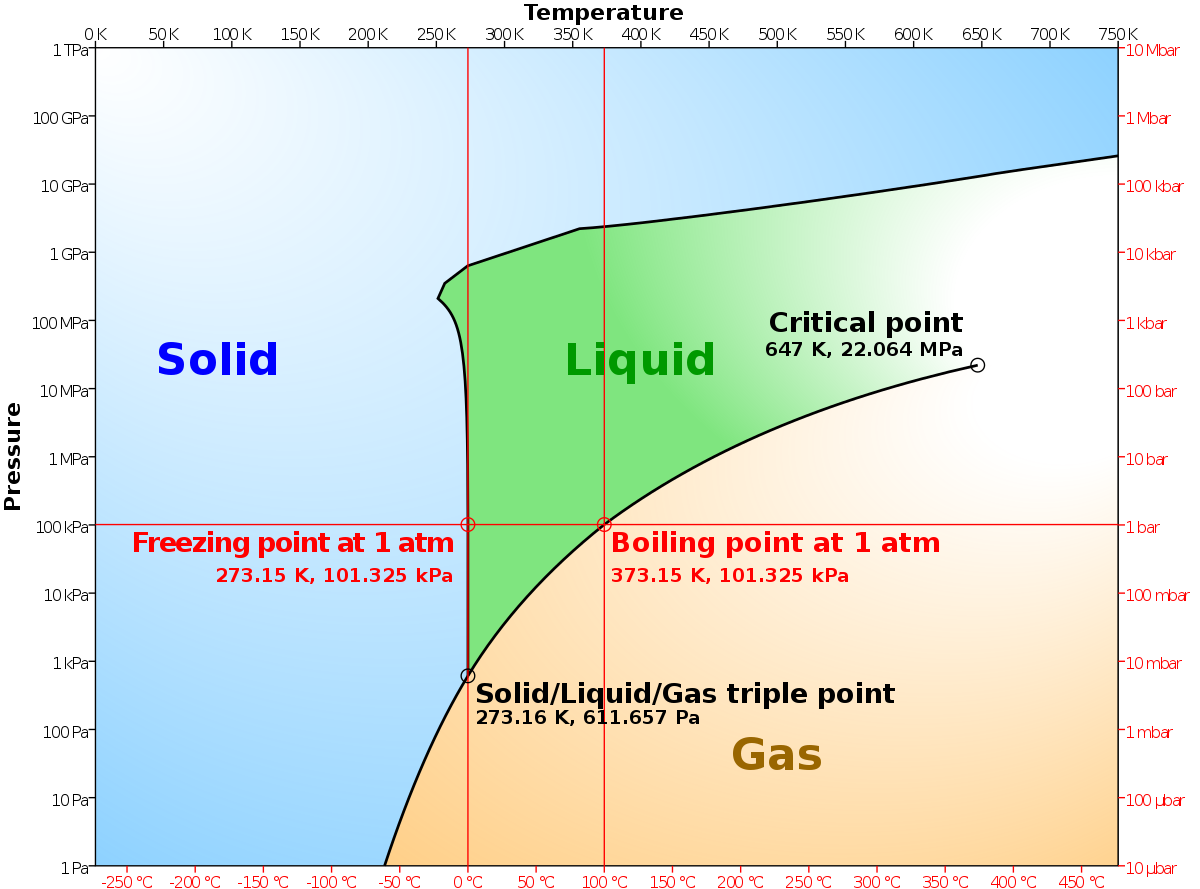
DOCX GitHub Pages The diagram shows the distribution of energy for the molecules in a sample of gas at a given temperature, T1 (a) In the diagram . E. a. represents the . activation energy . for a reaction. Define this term. (b) On the diagram above draw another curve to show the energy distribution for the same gas at a higher temperature. Label the curve T. 2. (c) Will mark as brainliest. this diagram would represent the ... In the diagram shown above, abcd is a parallelogram. the ratio of the area of triangle agb to the area of triangle cge is 9: 25. if cg=10 and ge=15 find ag. Answers Mathematics, 25.06.2019 17:10 Hess's Law and enthalpy change calculations - chemguide Enthalpy change is simply a particular measure of energy change. You will remember that the enthalpy change is the heat evolved or absorbed during a reaction happening at constant pressure. I have labelled the vertical scale on this particular diagram as enthalpy rather than energy, because we are specifically thinking about enthalpy changes.
This diagram would represent the enthalpy change in which of the following. Quiz & Worksheet - Drawing & Labeling Enthalpy Diagrams ... How to Draw & Label Enthalpy Diagrams - Quiz & Worksheet. Choose an answer and hit 'next'. You will receive your score and answers at the end. Glucose + Oxygen goes to carbon dioxide and water ... The diagram illustrates the enthalpy changes of a set of ... Click here👆to get an answer to your question ️ The diagram illustrates the enthalpy changes of a set of reactions.Which statements are correct? 1 . The enthalpy change for the transformation U → R is + 42 kJ mol^-1 2 . The enthalpy change for the transformation T → S is endothermic. 3 . The enthalpy change for the transformation R → T is - 33 kJ mol^-1 . Week7 Workshop.pdf - CHEM1100 Chemistry 1 2021 Workshops ... CHEM1100 Chemistry 1 2021 Workshops Task A: Understanding Enthalpy Changes Introduce yourself to the people at your table (or in your breakout room). A reaction profile (coordinate) diagram represents the relative energies for the reactants and products as the reaction progresses. The difference between the total potential energy for the reactants and the total potential energy for the ... Study 16 Terms | sem 2 chem 4.06 Flashcards - Quizlet there must be a change in enthalpy that is "hidden," or not reflected by a change in temperature. ... What is enthalpy? the heat content of a system at constant pressure. Which of the following symbols represents enthalpy? H. The latent heat in a substance has a negative value. Which of the following situations could be true? ... This diagram ...
Thermodynamics Conceptual | Chemistry Quiz - Quizizz Q. CO (g) + 2 H 2(g) ↔ CH 3 OH (g) K = 2.2 x10 4 at 298 K. A stoichiometric mixture of CO (g) and H 2(g) was allowed to react in two different rigid containers at a constant temperature of 298 K. The reaction is represented by the equation above. Diagram 1 represents the uncatalyzed reaction and diagram 2 represents the catalyzed reaction one hour after the reactants were mixed. Which of the following does not represent enthalpy change ... Enthalpy of formation is the heat change when one mole of compound is obtained from its constituent elements. Thus, it does not represent enthalpy change during phase transformation. While enthalpy of fusion, vapourization and sublimation represents enthalpy change during transformation from solid to liquid, liquid to gas and solid to gas ... enthalpy | Chemistry Quiz - Quizizz "Heat change when 1 mole of gaseous atom is formed from its element at standard states" is the definition for _____. answer choices standard enthalpy of formation This diagram would represent the enthalpy changes in which ... This diagram would represent the enthalpy changes in which of the following? - 9007542 bmisses bmisses 03/03/2018 Chemistry College answered This diagram would represent the enthalpy changes in which of the following? boiling liquid hot pack cold pack melting solid 1 See answer bmisses is waiting for your help. Add your answer and earn points.
PDF ENTHALPY 3. The enthalpy change for the following reaction: H 2 (g) + I 2 (g) 2HI(g) is -9 kJmol-1 Use this information and the values in the above table to calculate the bond dissociation energy for the H-I bond. 4. Explain why using bond dissociation energies only give you an approximate value for the overall energy change Measuring enthalpy changes 6. Reaction Coordinate Diagram - VIZISCIENCE® INTERACTIVE LABS 6. Reaction Coordinate Diagram. Given the following reaction, sketch a reaction coordinate graph. The reaction involves two steps, step 1 is the slowest step and step 2 is the fastest step. Both steps are exothermic. Indicate on the diagram the overall enthalpy change of the reaction, the reaction for the transition states and intermediate states. 3:04 calculate the molar enthalpy change (ΔH) from the ... 3:04 calculate the molar enthalpy change (ΔH) from the heat energy change, Q 3:05 (Triple only) draw and explain energy level diagrams to represent exothermic and endothermic reactions 3:06 (Triple only) know that bond-breaking is an endothermic process and that bond-making is an exothermic process Represent the potential energy/enthalpy change in the ... Represent the potential energy/enthalpy change in the following processes graphically. (a) Throwing a stone from the ground to roof. (b) 1/2 H 2 (g) + 1/2 Cl 2 (g) ⇄ HCl(g) Δ r H Θ = -92.32 kJ mol -1 . In which of the processes potential energy/enthalpy change is contributing factor to the spontaneity?
How to Calculate Enthalpy Change - Sciencing When a substance changes from solid to liquid, liquid to gas or solid to gas, there are specific enthalpies involved in these changes. The enthalpy (or latent heat) of melting describes the transition from solid to liquid (the reverse is minus this value and called the enthalpy of fusion), the enthalpy of vaporization describes the transition from liquid to gas (and the opposite is ...
Solved The following Lewis diagram represents the valence ... The following Lewis diagram represents the valence electron configuration of a main-group element. (Enter the answer using superscript.) If this element is in period 4, its valence electron configuration is pt Using average bond enthalpies (linked above), estimate the enthalpy change for the following reaction: 2HCl (g) + Brz (9) >2HBr (g ...
PDF Section A - Weebly AS-Level ENERGY CHANGES Q16 Given the following enthalpy changes, What is the standard enthalpy change of formation of iodine trichloride, ICl3(s)? A +176 kJ mol-1 . B -88 kJ mol-1 . C -176 kJ mol -1 . D -214 kJ mol -1 . Q17 Hydrogen peroxide slowly decomposes into water and oxygen. The enthalpy change of
PDF Lattice Enthalpy NEW - WordPress.com represent them. 1) The enthalpy change of formation Δ fH ө the enthalpy change associated with the formation of one mole of a substance from its elements in their standard states under standard conditions of 298K and 101kPa The is usually an EXOTHERMIC process for an ionic compound. e.g. K (s) + ½ Cl 2(g)! KCl (s) Δ f H ө = -437 kJ mol-1
Chemistry 4.06 Quiz: Changes in Enthalpy Cheat Sheet ... This diagram would represent the enthalpy change in which of the following? liquid water freezing A 10 g gold coin is heated from 25°C to 50°C (CAu is 0.13 J/g-°C).
PDF Enthalpy diagram & energy stoichiometry Potential Energy diagrams and Energy Stoichiometry Use the diagram to answer questions 1-5. .Which letter represents the activation energy for the reaction? 2.Which letter represents the enthalpy change for the reaction? 3.1s the reaction in the diagram exothermic or endothermic? 4.1s the enthalpy change positive or negative?
Solved Which of the following statements concerning ... a) The activation energy of a reaction is proportional to its enthalpy change b) The activation energy is measured from the origin to the top of the peak c) The activation energy of the reverse reaction may be calculated from the diagram d) The peak on this diagram represent an ineffective collision e) The units of the diagram are joules and ...
How can I represent enthalpy in a potential energy diagram ... This enthalpy is the difference in energy between the nonbonded state and the bonded state, and in the diagram, is therefore in reference to the zero-point energy. In this case, the zero-point energy represents no bonding interactions.
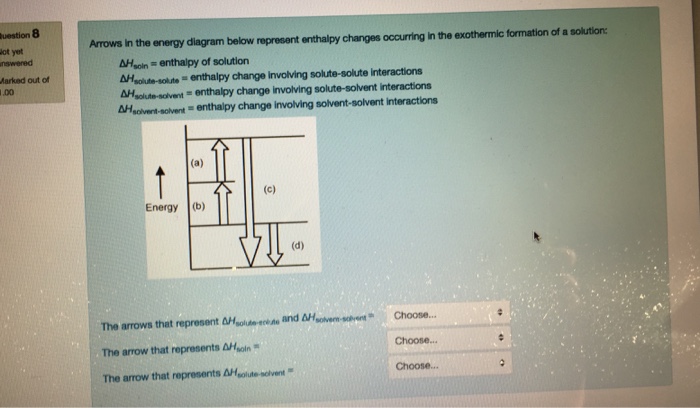
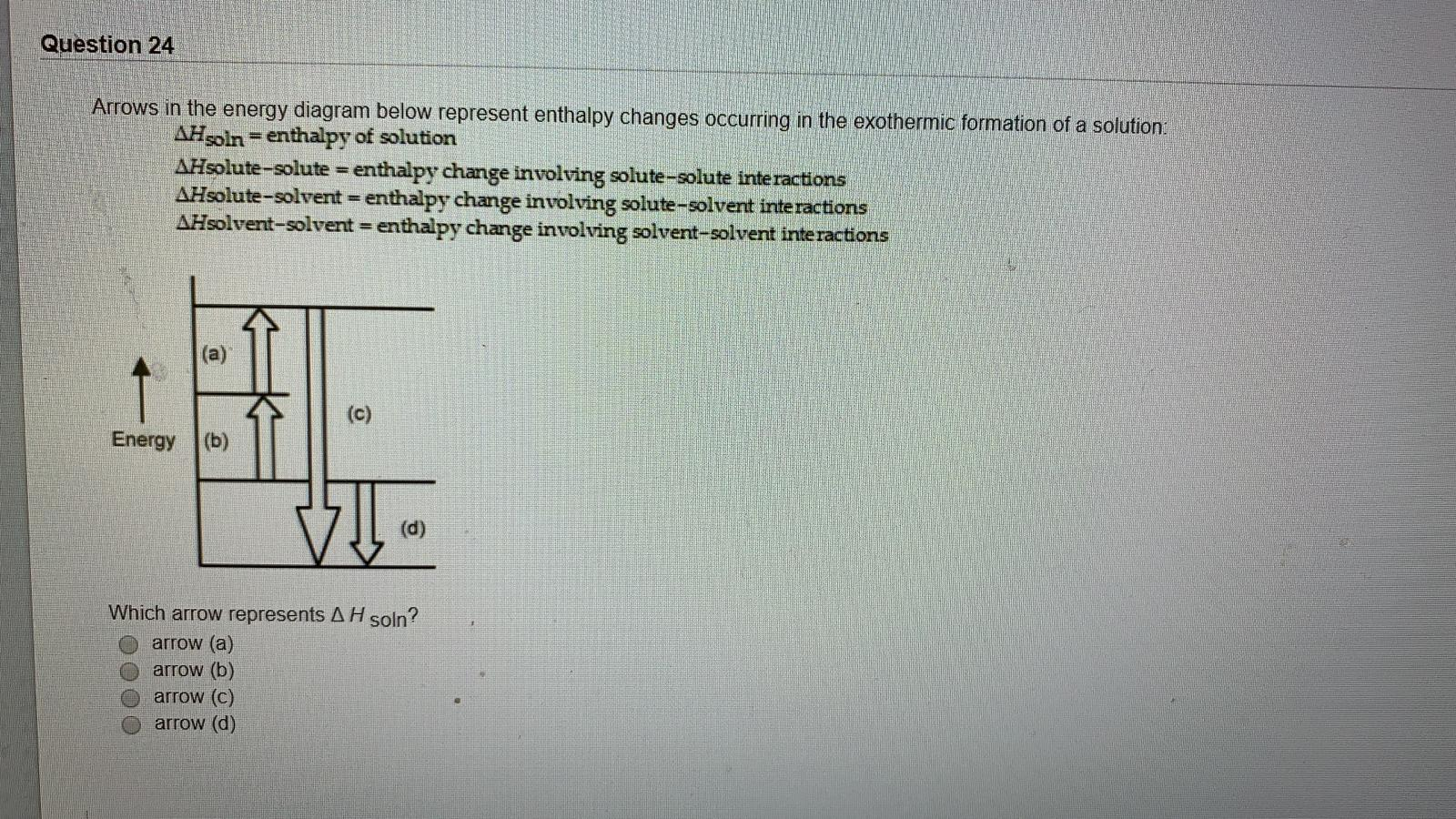
Arrows in the energy diagram below represent enthalpy ... Iron oxide reacts with aluminum in an exothermic reaction. Fe2O3(s) + 2Al(s) -> 2Fe(s) + Al2O3(s) The reaction of 5.00 g Fe2O3 with excess Al evolves 26.6 kJ of energy in the form of heat. Calculate the enthalpy change per mole of Fe2O3 reacted
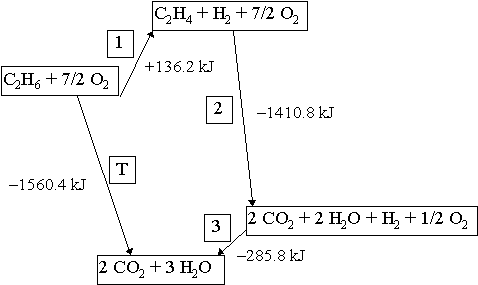
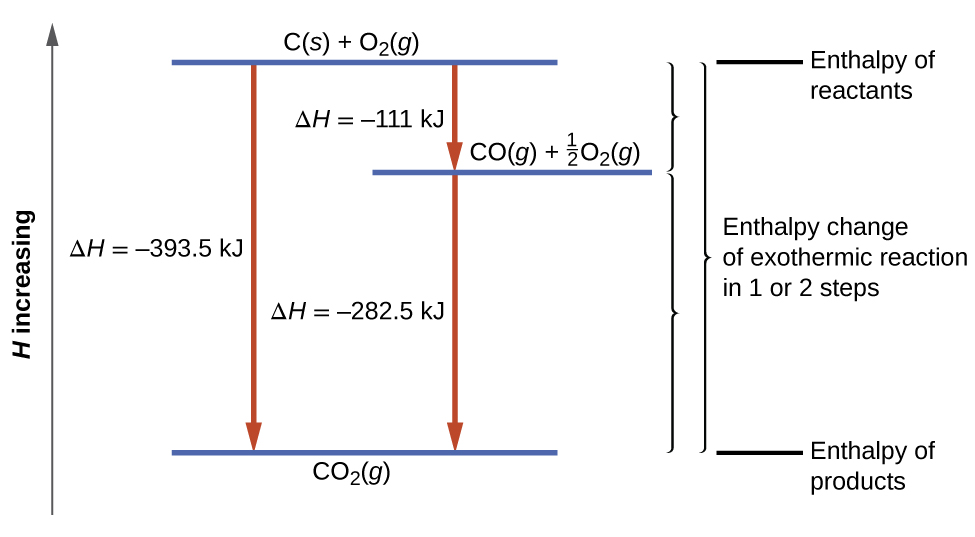
Energetics: 4.32 - Hess' law energy cycles and diagrams The enthalpy level diagram can now be constructed. In this case, the red dotted line is obtained by subtracting the small blue dotted line from the longer blue dotted line. That is, the enthalpy of 4 - 2 = -463 - (-124) = -339 kJ. Therefore the standard enthalpy of formation, ΔH f, of phosphorus(III) chloride = -339 kJ
Hess's Law and enthalpy change calculations - chemguide Enthalpy change is simply a particular measure of energy change. You will remember that the enthalpy change is the heat evolved or absorbed during a reaction happening at constant pressure. I have labelled the vertical scale on this particular diagram as enthalpy rather than energy, because we are specifically thinking about enthalpy changes.
Will mark as brainliest. this diagram would represent the ... In the diagram shown above, abcd is a parallelogram. the ratio of the area of triangle agb to the area of triangle cge is 9: 25. if cg=10 and ge=15 find ag. Answers Mathematics, 25.06.2019 17:10
DOCX GitHub Pages The diagram shows the distribution of energy for the molecules in a sample of gas at a given temperature, T1 (a) In the diagram . E. a. represents the . activation energy . for a reaction. Define this term. (b) On the diagram above draw another curve to show the energy distribution for the same gas at a higher temperature. Label the curve T. 2. (c)




















0 Response to "37 this diagram would represent the enthalpy change in which of the following"
Post a Comment