37 in an electron dot diagram of ethylene how many double bonds are present
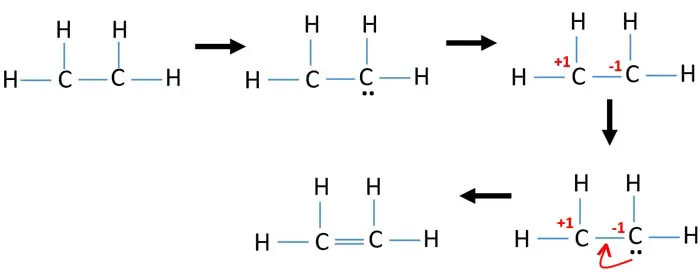
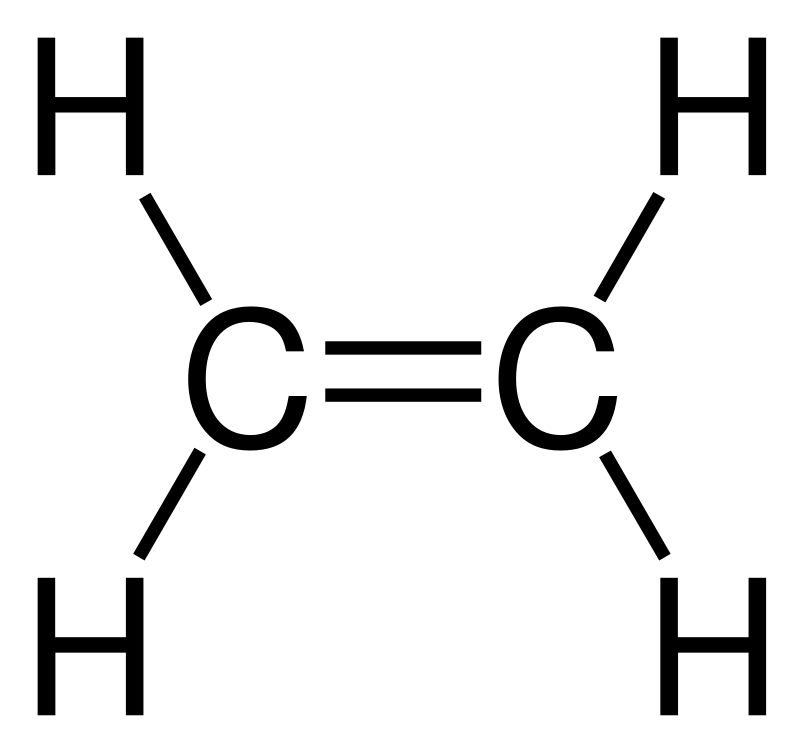
In an electron dot diagram of ethylene C2H4 how many double ... Ethylene has 4 single bonds (carbon to hydrogen) and 1 double bond (carbon to carbon). What is the full structural formula for CH2-CH2 in ethylene? how many c2h4 molecules are contained in 45.8 - Lisbdnet.com How many electron groups are in C2H4? As per the C2H4 Lewis structure, Four C-H sigma bonds are present and one C=C double bond(1 sigma + 1 pie bond). It means four C-H bond has 8 shared pairs of electrons and C=C bond has 4 shared pairs of electrons. Hence total shared pairs of electrons in the dot diagram of C2H4 is 12.
how many electron pairs are shared between the carbon ... Explanation: That's ethylene and it just have one double bond. You can know if it's a simple, double or triple bond by the number of hydrogens: C2H6 :three hydrogens per carbon, ethane, simple bond; C2H4 : two hydrogens per carbon, ethylene, double bond; C2H2 : one hydrogen per carbon, acetylene, triple bond.

In an electron dot diagram of ethylene how many double bonds are present
38 in an electron dot diagram of ethylene how many double ... A Double bond is when two atoms share two pairs of electrons with each other. 4.17 Chemistry Unit Assessment K12 Flashcards - Quizlet In an electron dot diagram of ethylene (C2H4) how many double bonds are present?... Which type of bond in ethylene? Score: 4.6/5 (42 votes) . Ethylene (commonly knows as ethene), CH 2 CH 2, is the simplest molecule which contains a carbon carbon double bond.The Lewis structure of ethylene indicates that there are one carbon-carbon double bond and four carbon-hydrogen single bonds. Sigma and Pi Bonds | Chemistry for Non-Majors In a conventional Lewis electron-dot structure, a double bond is shown as a double dash between the atoms as in C=C. It is important to realize, however, that the two bonds are different: one is a sigma bond, while the other is a pi bond. Ethyne (C 2 H 2 ) is a linear molecule with a triple bond between the two carbon atoms (see Figure 4).
In an electron dot diagram of ethylene how many double bonds are present. In an electron dot diagram of ethylene (c2h4), how many ... SHOW ANSWER. Ethylene, also known as ethene, has two carbon atoms and four hydrogen atoms. The carbon atoms in ethene exhibit a valency of 4; therefore, in order to complete their outermost shell, the carbon atoms form a double bond with one another. Thus, the number of double bonds present in a molecule of ethylene is 1. The number of covalent bonds in ethylene is: - Toppr 1 double and 5 single bonds Medium Solution Verified by Toppr Correct option is D) Ethene means C2H4 and it is an alkene. The total number of covalent bonds in the molecule of ethene will be 6 ( one sigma bond and one pi bond between the two carbon atoms and 4 hydrogen atoms remain attached to the two carbon atoms by 4 sigma bonds ) CH 2 =CH 2 K12. 4.17 Unit Assessment: Chemical Bonding, Part 1. 2018 ... In an electron dot diagram of ethylene (C2H4), how many double bonds are present? One. What is the molecular shape of silicon tetrabromide? Tetrahedron. Which of the following intermolecular forces plays a pivotal role in biological molecules such as proteins and DNA? Hydrogen bonding. Draw Lewis structure of tetracyanoethylene and point class ... The structure of tetracyanoethylene molecule is: Here, as you can see, there are nine sigma bonds and nine pi bonds. The nine sigma bonds consist of four between the four C ≡ N groups, and four between the C − C bond attached to cyanide and one sigma bond of ethylene, whereas there are nine pi bonds. Two pi bonds lie between each C ≡ N (cyanide) and therefore it will be eight pi bonds and one bond lies between C = C bond.
Ethene (C2H4) lewis dot structure, molecular geometry ... As per the C2H4 Lewis structure, Four C-H sigma bonds are present and one C=C double bond(1 sigma + 1 pie bond). It means four C-H bond has 8 shared pairs of electrons and C=C bond has 4 shared pairs of electrons. Hence total shared pairs of electrons in the dot diagram of C2H4 is 12. covalent bonding - double bonds - chemguide Two oxygen atoms can both achieve stable structures by sharing two pairs of electrons as in the diagram. The double bond is shown conventionally by two lines joining the atoms. Each line represents one pair of shared electrons. Carbon dioxide, CO 2. Ethene, C 2 H 4. Ethene has a double bond between the two carbon atoms. C2H4 Lewis Structure, Molecular Geometry, Hybridization ... Since there are two bonds forming here, we will have a double bond structure. Hence, C2H4 is an alkene. Here, we have got the most suitable and appropriate Lewis Structure Sketch of ethylene. Molecular Geometry When we draw the Lewis Structure of C2H4, we find a linear 2-D representation. In reality, the molecular shape of ethene is not linear. which of the following ions will most likely ... - Brainly.com Ethylene has four single covalent bonds and one double covalent bond. The Lewis dot structure is used to show the bonding between the valence electrons of a compound. Ethylene has 12 valence electrons and these are shared in this manner: 4 [double bond], 2 [single bond], 2, 2, 2.
How many sigma and bonds are present in a molecule of ... The bond consists of two electron clouds which lie above and below the plane of carbon and hydrogen atoms. (a) Formation of ethylene (b) Molecular orbital structure molecule of ethylene Thus, ethylene molecule consists of four sigma C - H bonds, one sigma C - C bond and one bond between carbon-carbon atom. The bond length of carbon-carbon ... Quick Answer: How Many Pairs Of Electrons Are Shared In ... Ethylene, C2H4, is a chemical compound composed of two carbon atoms and four hydrogen atoms. What do you call the bond having 2 shared electron? A Double bond is when two atoms share two pairs of electrons with each other. It is depicted by two horizontal lines between two atoms in a molecule. Write the electron dot structure of ethene molecule (C2H4) Click here👆to get an answer to your question ️ Write the electron dot structure of ethene molecule (C2H4) . 4.16 Unit Test: Chemical Bonding Flashcards | Quizlet Z+. Which property is a characteristic of an ionic compound? high melting point. Hydrogen bonds can be found between molecules of which substance? NH3. Which compound contains a triple bond? acetylene (C2H2) In an electron dot diagram of ethylene (C2H4), how many double bonds are present? one.
PDF 3.4 Covalent Bonds and Lewis Structures - Columbia University If an atom lacks an octet, use electron pairs on an adjacent atom to form a double or triple bond. • Example: All the atoms have octets in this Lewis structure. Table 1.4 How to Write Lewis Structures.... H C O N O H H..:..
In an electron dot diagram of ethylene (C2H4), how many ... Ethylene, also known as ethene, has two carbon atoms and four hydrogen atoms. The carbon atoms in ethene exhibit a valency of 4; therefore, in order to complete their outermost shell, the carbon atoms form a double bond with one another. Thus, the number of double bonds present in a molecule of ethylene is 1. Advertisement Answer 4.8 /5 20 Megadeth
how many electron pairs are shared between the carbon ... As per the C2H4 Lewis structure, Four C-H sigma bonds are present and one C=C double bond(1 sigma + 1 pie bond). It means four C-H bond has 8 shared pairs of electrons and C=C bond has 4 shared pairs of electrons. Hence total shared pairs of electrons in the dot diagram of C2H4 is 12. How many electrons does ethene have?
Is C2H4 a double bond? - Answers C2H4, or ethylene has a double bond between the two carbon atoms. The hydrogen atoms are singly bonded at an angle of 121 degrees from the carbon bonding.
Chapter 8 - Chemical Bonds - CHE 105/110 - Introduction to ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. ... Double bonds or triple bonds between atoms may be necessary to properly ...
Ethylene | CH2=CH2 - PubChem The rate constant for the gas-phase reaction of ethylene with photochemically-produced hydroxyl radicals is 7.9X10-12 cu cm/molecule-sec at 25 °C (1). This corresponds to an atmospheric half-life of about 2 days at an atmospheric concentration of 5X10+5 hydroxyl radicals per cu cm (2).
How do you know how many bonds are in a Lewis structure? The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus the number of valence electrons. This method works because each covalent bond that an atom forms adds another electron to an atoms valence shell without changing its charge.
Draw the Lewis structure for the molecule CH2CHCH3 class ... Explanation: Lewis-dot structure: It shows the bonding between the atoms of a molecule as shown in the structure and it also shows the unpaired electrons present in the molecule. In the Lewis-dot structure the valence electrons are represented by 'dot'. The given molecule is propylene, C H 2 C H C H 3. . As we know that carbon has four valence ...
Covalent Bond - Definition, Types, Properties, and Examples Ethylene Molecule: In ethylene, each carbon atom shares two of its valence electron with two hydrogen atoms and remaining two electrons with the other carbon atom. So there is a double bond between the carbon atoms. Double Bond in Ethylene Molecule Triple Bond
Sigma and Pi Bonds | Chemistry for Non-Majors In a conventional Lewis electron-dot structure, a double bond is shown as a double dash between the atoms as in C=C. It is important to realize, however, that the two bonds are different: one is a sigma bond, while the other is a pi bond. Ethyne (C 2 H 2 ) is a linear molecule with a triple bond between the two carbon atoms (see Figure 4).
Which type of bond in ethylene? Score: 4.6/5 (42 votes) . Ethylene (commonly knows as ethene), CH 2 CH 2, is the simplest molecule which contains a carbon carbon double bond.The Lewis structure of ethylene indicates that there are one carbon-carbon double bond and four carbon-hydrogen single bonds.
38 in an electron dot diagram of ethylene how many double ... A Double bond is when two atoms share two pairs of electrons with each other. 4.17 Chemistry Unit Assessment K12 Flashcards - Quizlet In an electron dot diagram of ethylene (C2H4) how many double bonds are present?...








/ethylene-56a12bda5f9b58b7d0bcbafc.jpg)
















0 Response to "37 in an electron dot diagram of ethylene how many double bonds are present"
Post a Comment