37 ti2+ orbital diagram
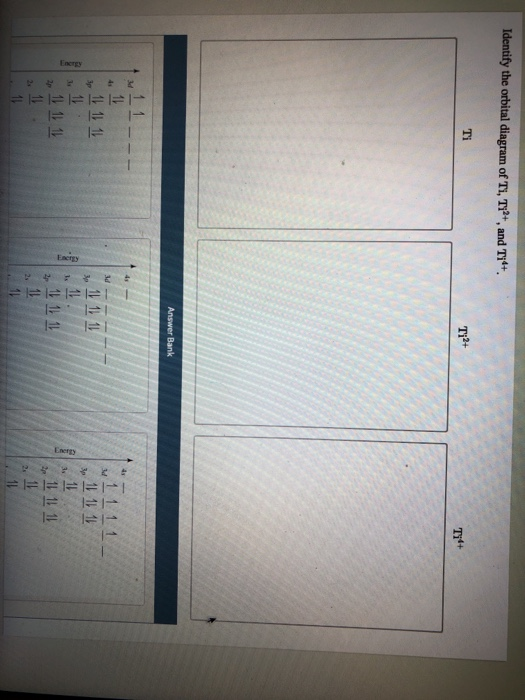
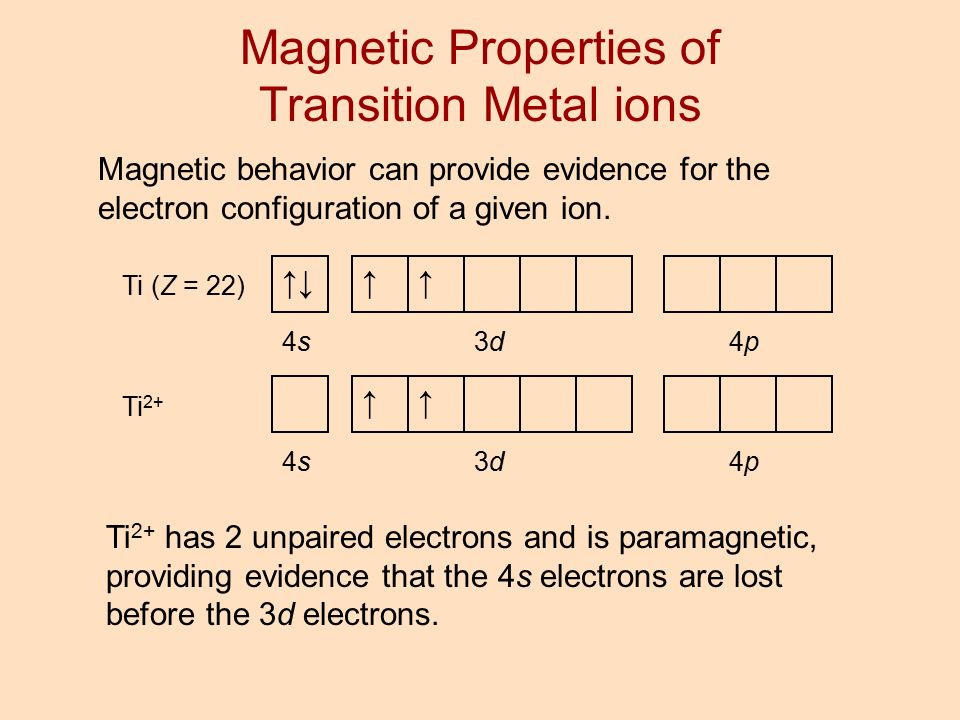
Orbital Diagram Of Ti2+ Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . Are all atoms with an odd number of electrons paramagnetic? If an atom has an odd number of electrons, it will be paramagnetic. But if it has an even number of electrons, you have to check if every electron is paired or not within its orbital. Example: Cr2+ has 22 electrons but look at its noble gas configuration: [Ar] 3d4.
The atomic orbital of the O atoms overlap to form the sigma and pie orbital of the O2 molecules as shown in the diagram above. We add the 12 valence electron according to the aufbau principle. The last two electrons go into separate degenerate pie orbital. According to hund's rule. Thus,oxygen has two unpaired electrons and is paramagnetic.
Ti2+ orbital diagram
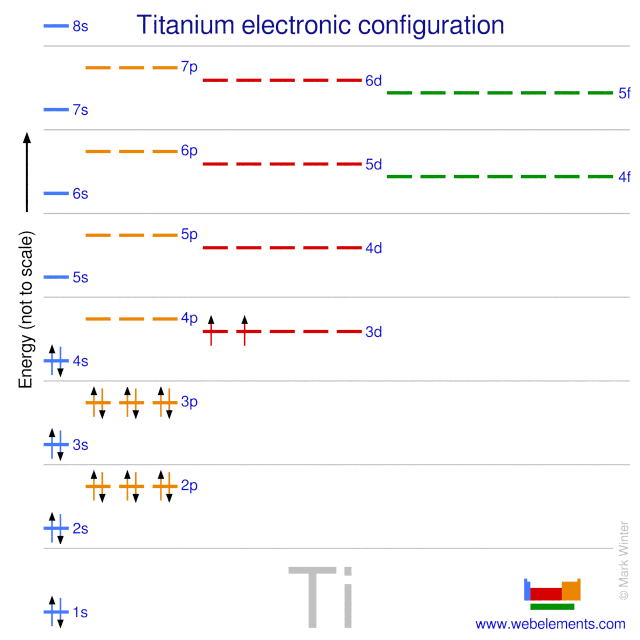
on Orbital Diagram For Ti2+. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0. However, once the 4s orbital is filled, it becomes higher in energy than the 3d orbitals. This means that when titanium loses electrons, it does so. These velocities are related as shown in the diagram at the right. (a) (b) Since v we is vertical, v wc sin 60.0° = v ce = 50.0 km h or v wc = 57.7 km h at 60.0° west of vertical . Since v ce has zero vertical component, b vce vwe 60° vwc v we = v ce + v wc FIG. P4.39 g v we = v wc cos 60.0° = 57.7 km h cos 60.0° = 28.9 km h downward . Chapter 4 P4.40 The bumpers are initially 100 m … Research in the IDM is led by over 34 independent principal investigators in the basic, clinical and public health sciences, and has a strong translational focus. Grant and contract funding is sourced from the US National Institutes of Health, the Bill & Melinda Gates Foundation, The Wellcome Trust, EDCTP, the South African Medical Research Council, the National Research Foundation …
Ti2+ orbital diagram. Titanium (Ti) excited state electron configuration and orbital diagram When a titanium atom is excited, then the titanium atom absorbs energy. As a result, an electron in the 4s orbital jumps to the 4px sub-orbital. Therefore, the electron configuration of titanium (Ti*) in excited state will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d xy1 3d yz1 4s 1 4p x1. diagram below shows a length of dna containing a bacterial gene drag the labels to their appropriate locations in the diagram to describe the function or characteristics of each part of the gene, answer to construct the orbital diagram of each atom or ion ti ti2 ti4, note you could have just plugged the To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number... What is the correct electron configuration for Ti2 +? Note that the 4s electrons are removed before the 3d electrons, so the electron configuration of Ti2+ would be [Ar]3d2 not [Ar]4s2. 8. What are the properties of CD? Cadmium is a lustrous, silver-white, ductile, very malleable metal.
This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble... 27 Jun 2016 — Now, it's important to keep in mind that this notation for the electron ... However, once the 4s orbital is filled, it becomes higher in ...1 answer · Ti2+:[Ar]3d2 Explanation: A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the ... by A Kalemos · 2011 · Cited by 21 — electrons, the combined orbital and spin angular momenta ... Relative energy level diagram of Ti2 of 30 states at the MRCI+Q/Qζ.9 pages watching. 726. views. greenkangaroo798 Lv1. 28 Nov 2020. What is the orbital diagram of each atom or ion? Ti, Ti 2+, Ti 4+. Answer. + 20.
See the answer See the answer done loading. Construct the orbital diagram of each atom or ion. Ti. Ti2+. Ti4+. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Construct the orbital diagram of each atom or ion. Ti. Ti 2 Oct 15, · Normally you would expect the electron configuration of Ti to be [Ar] 4s23d2, but in actuality it is 4s13d3. If you took two electrons away due to the +2 charge, you would have 4s13d1. 19.03.2021 · Polarons are quasiparticles that easily form in polarizable materials due to the coupling of excess electrons or holes with ionic vibrations. These quasiparticles manifest themselves in many ... Tata Mcgraw-HillThe orbital diagram for Mn2 + is Ti2 + 3d2 V2 + 3d Cr3 + 个个个 3d [ Ar ] T111 T Mn2 + 3d 个个个( ii ) [ Fe ( H20 ) 6 ] 2+ , u = 5.3 BM corresponds to 4 ...
tipped pcd inserts with brazed pcd cutting edges are used for machining non-ferrous hard metals such as aluminum alloy, tungsten carbide, copper, zinc. the working tips of pcd inserts are made by pure polycrystalline diamond, pcd tipped inserts are mainly for cnc continuous turning and milling automobile engine block, cylinder head, transmission parts, gearbox.
What is the orbital diagram for Ti 2+? I got 1s two arrows, 2s two arrows, 2p 6 arrows, 3s two arrows, 3p six arrows, 4s two arrows but it is wrong. It said ions of d-block metals typically lack the outermost s electrons that are present in their neutral counterparts.
Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . Which free ion has the greater number of unpaired d electrons, Ti2+ or Co2+? Draw the orbital diagram for the d orbitals in an octahedral complex containing.
Answer to Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+.
04.07.2014 · The space-time diagram of Fig. 1.93 shows three events A, B, and C which occurred on the x axis of some inertial reference frame. Find: …
Orbital Diagram for Ti2. electron configuration for the titanium ion ti2 this video shows you how to write the electron configuration for the titanium ion ti 2 electron orbitals question 111 so if you count the electrons of the ti2 in the s orbital you need to know hund s rule and specifically this diagram electron orbitals.
Now if it's Ti4+, now we've taken away two additional electrons compared to the Ti2+. So we would write out 1s2, 2s2, 2p6, 3s2, 3p6, and then the 3d2-electrons would be gone. So compared to the original Titanium atom, we gave away four electrons. So both the 4s2, and the 3d2 electrons would be gone.
Section 1.6 - 4 • A general term symbol that uniquely describes a specific electronic configuration looks like this: (2S+1)L J where 2S + 1 is the spin multiplicity (and S is the total spin angular momentum.) L is the total orbital angular momentum J is the total angular momentum (spin + orbital) S = 0 → "Singlet" S = ½ → "Doublet" S = 1 → "Triplet" etc.
Choose the ground state electron configuration for Ti2+ ... Choose the valence electron orbital diagram that represents the ground state of Se2-D (#1) The solid compound K2S2O3 contains. K+ ions and S2O3^2-Of the following, which atom has the largest atomic radius? K. Choose the best Lewis structure for SF4. E (#28)
Problem Details. Construct the orbital diagram of each atom or ion. Ti. Ti 2+. Ti 4+. Learn this topic by watching The Electron Configuration: Ions Concept Videos.
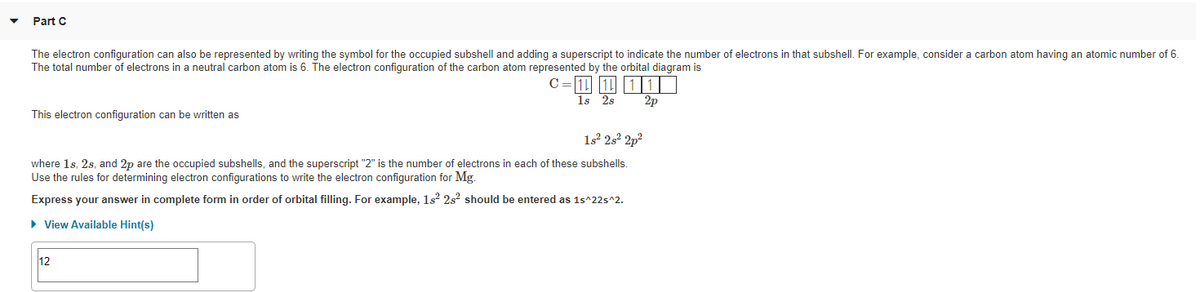
The two electrons that are lost when the Ti2+ is formed will come from the 4s orbital, which means that the electron configuration of the cation is Ti2+:1s2 ...1 answer · Top answer: The electron configuration of a neutral titanium atom is Ti: 1s2 2s2 2p6 3s2 3p6 4s2 3d2 The two electrons that are lost when the Ti2+ is formed will come ...
Electron Configuration Of Oxygen - 9 images - ionization of water using the e 2e technique from the, molecular orbital diagrams for o2,
Solutions Manual Fundamentals of Analytical Chemistry 9th Edition
Research in the IDM is led by over 34 independent principal investigators in the basic, clinical and public health sciences, and has a strong translational focus. Grant and contract funding is sourced from the US National Institutes of Health, the Bill & Melinda Gates Foundation, The Wellcome Trust, EDCTP, the South African Medical Research Council, the National Research Foundation …
These velocities are related as shown in the diagram at the right. (a) (b) Since v we is vertical, v wc sin 60.0° = v ce = 50.0 km h or v wc = 57.7 km h at 60.0° west of vertical . Since v ce has zero vertical component, b vce vwe 60° vwc v we = v ce + v wc FIG. P4.39 g v we = v wc cos 60.0° = 57.7 km h cos 60.0° = 28.9 km h downward . Chapter 4 P4.40 The bumpers are initially 100 m …
on Orbital Diagram For Ti2+. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0. However, once the 4s orbital is filled, it becomes higher in energy than the 3d orbitals. This means that when titanium loses electrons, it does so.



















0 Response to "37 ti2+ orbital diagram"
Post a Comment