40 lewis dot diagram for al
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions: (a) K and S (b) Ca and O (c) Al and N. Answer (a) K and S: The electronic configurations of K and S are as follows: K: 2, 8, 8, 1. S: 2, 8, 6. Sulphur (S) requires 2 more electrons to complete its octet. The valence electrons are written as dots surrounding the symbol for the element: one dot is place on each side first, and when all four positions are filled, the remaining dots are paired with one of the first set of dots, with a maximum of two dots placed on each side. Lewis-dot diagrams of the atoms in row 2 of the periodic table are shown ...
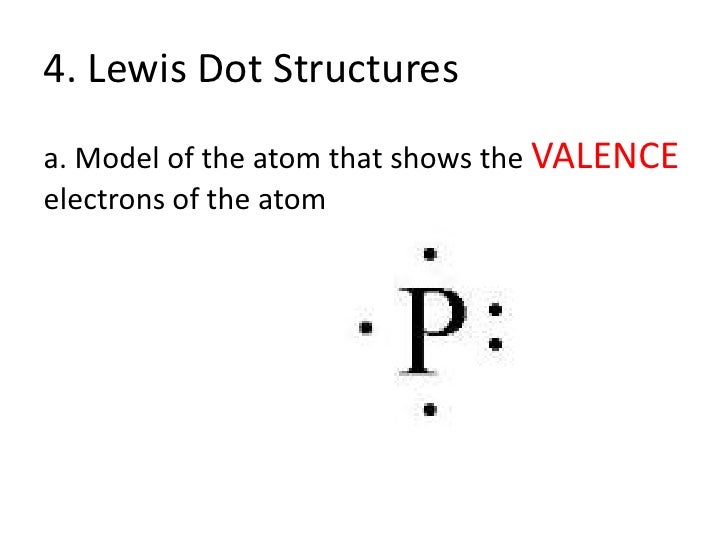
Dot Diagrams (sometimes known as Lewis dot diagrams) are a depiction of an atom's valence electrons. They are a powerful tool in helping you understand, see, and even predict molecular bonding. Ne 1 2 5 4 6 8 3 7 ... Al 6 C 32 Ge 14 Si 7 N 33 As 15 P 8 O 34 Se 16 S 9 F 35 Br 17 Cl 2 He 10 Ne 36 Kr 18 Ar Metals Non-Metals Dividing line

Lewis dot diagram for al
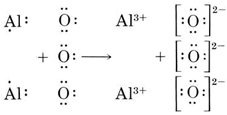
Here are a number of highest rated Al2o3 Lewis Dot Structure pictures upon internet. We identified it from well-behaved source. Its submitted by presidency in the best field. We recognize this nice of Al2o3 Lewis Dot Structure graphic could possibly be the most trending subject in imitation of we portion it in google pro or facebook. Aluminium is exceptional to the octet and it is also called octet deficient. But in the case of the AlCl3 lewis diagram, the Aluminium central atom get's a formal charge equal to zero when it has 6 electrons around. But when an aluminium central atom is distributed with 8 electrons it gets an uneven formal charge. A step-by-step explanation of how to draw the Lewis dot structure for Al (Aluminum). I show you where Aluminum is on the periodic table and how to determine...
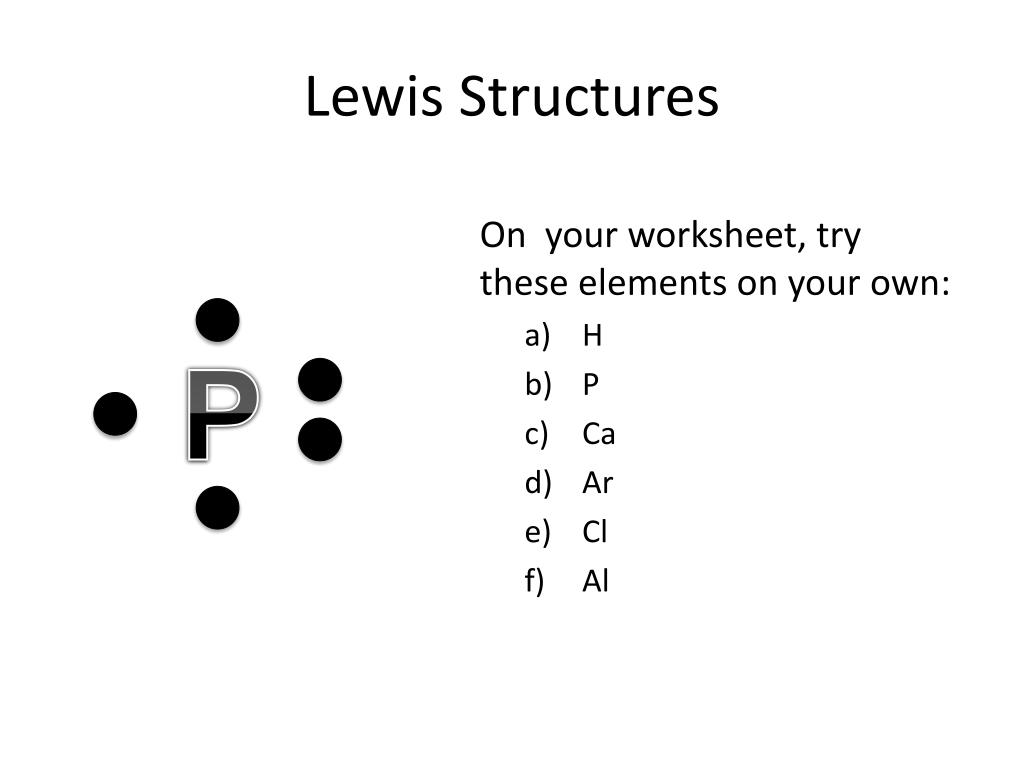
Lewis dot diagram for al. Lewis Dot Structure Worksheet with Answers - Worksheets make it possible for users strategies that are common and distinctive to filter and. Nov 14 30 00 GMT lewis dot diagrams chemistry handout pdf Honors. Chemistry is Lewis Dot Diagrams Name Chem Worksheet 5 7. Diagrams Chemistry Handout Answers Pdf, Read Online Lewis Dot Diagrams Chemistry. In the box below, draw a Lewis electron-dot diagram for a molecule of phosphorus trichloride, PC13 (c) ammonia 60) 61) 62) 65) In the box provided, draw a Lewis electron-dot diagram for a molecule of chlorine, Ch. in terms of electrons, why the bonding in NaCl is Ionic. In the box below, draw the electron-dot (Lewis) structure of hydrogen bromide. A simple notation used to represent valence electrons in an atom is called Lewis symbol. According to him, atoms achieve stable octet by gaining, loosing or ... To draw Lewis dot structures, start by writing the atomic symbols for the 2 atoms side-by-side. Then, determine whether the atoms are held together by a single, double, or triple bond. Next, draw lines between the atoms to represent that bond. For example, use 1 line to show a single bond, or draw 2 lines if they have a double bond.
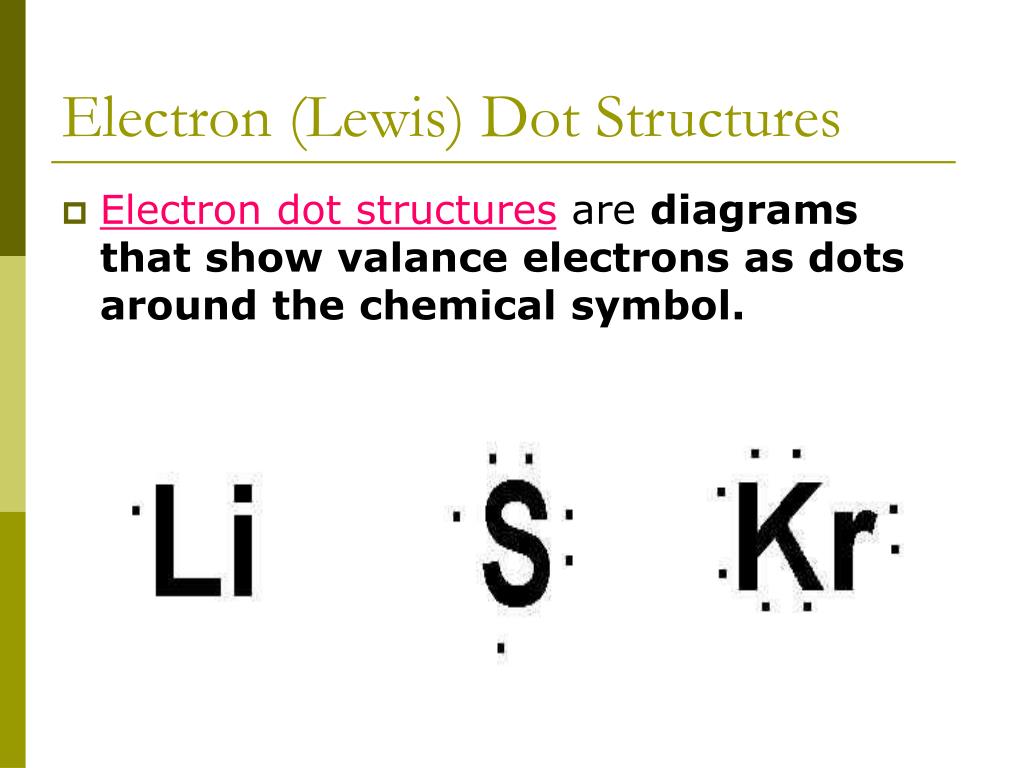
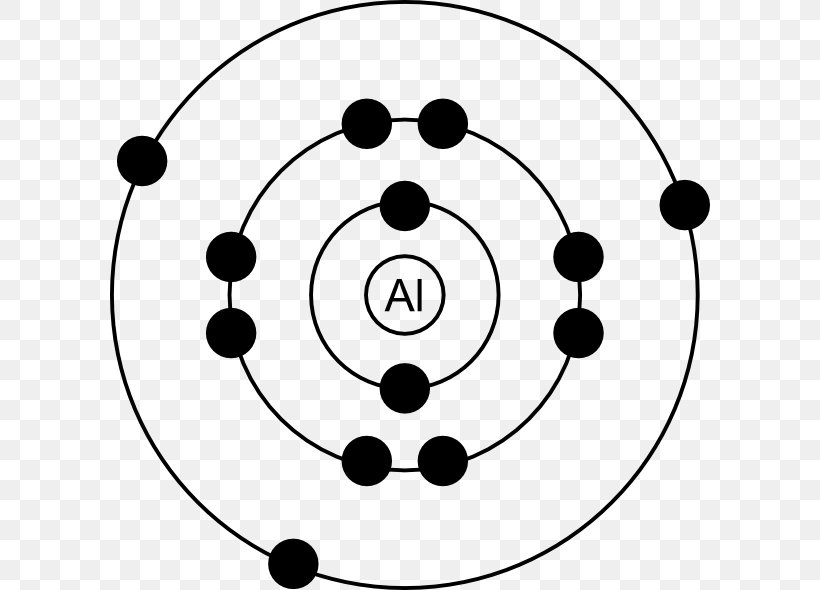
There is another model called the electron dot or Lewis diagram. This system represents an atom and its valence electrons. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. The electrons in the valence shell are shown as dots placed around the symbol. A Lewis dot diagram is a representation of an element surrounded by its valence electrons. The diagram consists of the element symbol (from the periodic table), with dots on the top, bottom, and sides representing the sand psub-levels of its valence shell. For example, aluminum has 3 valence electrons. The orbital-notation electron Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 4 dots around the symbol Lithium 3 7 3 3 Li
full electronic structure diagram of aluminium fluoride, the blue circle represents the nucleus. The electronic dot & cross diagram for the ionic bonding in the ionic compound aluminium fluoride the Lewis diagram for the formation of aluminium fluoride Melting point of aluminium fluoride is 1290oC Solid aluminium How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16 Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element. A step-by-step explanation of how to draw the AlF3 Lewis Dot Structure (Aluminum fluoride).For the AlF3 structure use the periodic table to find the total nu... A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms.
What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3 s2 3 p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons: The valence electron configuration for selenium is 4 s2 4 p4.
Feb 10, 2021 · Because it is part of team 3, it has actually 3 valence electrons. When drawing the Lewis structure for aluminum, location three points or valence electrons approximately the price (Al). You are watching: Draw the lewis dot structure for al. And what is the Lewis framework of Al? Answer: Aluminum belong to group IIIA that the routine table and also therefore has three valence electrons. The price of aluminum is Al, i m sorry is surrounded by 3 dots. 2.
Sep 14, 2011 · What is the Lewis dot diagram for al? In Lewis dot notation aluminum has three dots as it is group 13 and has 3 valence electrons.
A step-by-step explanation of how to draw the AlI3 Lewis Dot Structure.For the AlI3 structure use the periodic table to find the total number of valence elec...
Example 1: Writing Lewis DoT SYmbols of Elements · The valence electron configuration for aluminum is 3s 23p 1. So it would have three dots around the symbol for ...
Lewis Structure for CH 4 H H Repeat first two steps from before 1. Use the periodic table to decide how many electrons are around each atom 2. Write the electrons around each atom Example: Draw the Lewis Structure for CH 4 Remember, "H" can't go in the middle…put them around the Carbon! H C H Carbon has 4 electrons Each hydrogen has 1 H H
The Lewis Dot Diagrams for the corresponding elements are a useful way to show the arrangement of outer (valence) electrons on the atom. oxygen Atam Bohr Diagram 10 n Fluorine Atom Bohr Diagram lip 12 n Sodium Atom Bohr Diagram Group 16, VIA, Group 17, Lewis Svrnbol Lewis Symbol Group 1 or IA Lewis Symbol Bohr Notation 2-8-7 2-8-18-5 Lewis Dot
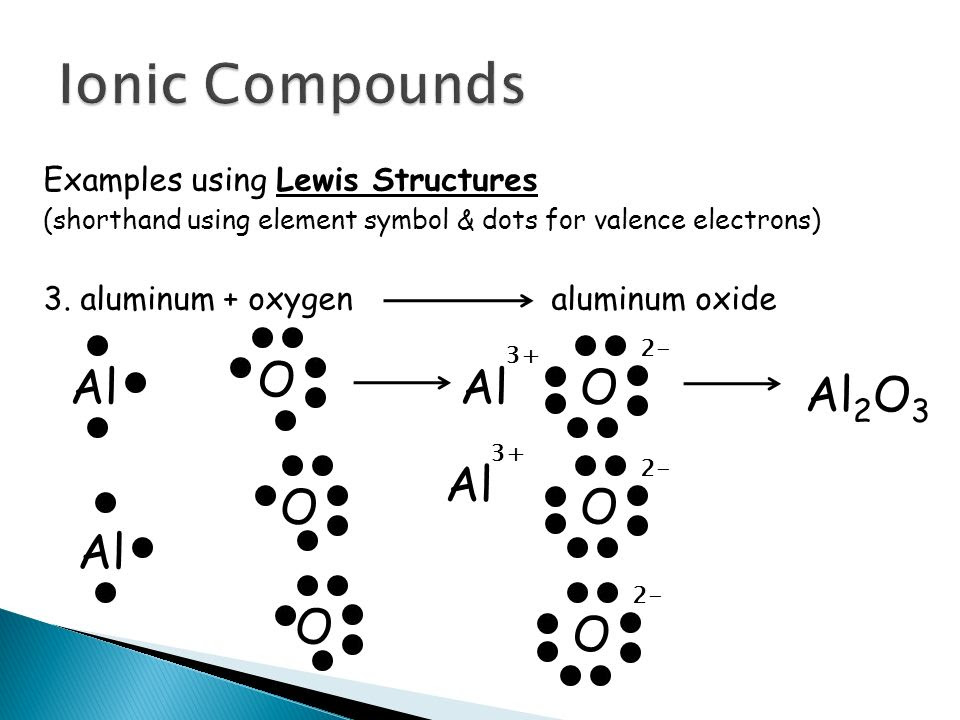
Write the Lewis symbols of the ions in each of the following ionic compounds and the Lewis symbols of the atom from which they are formed: (a) MgS (b) Al 2 O 3 (c) GaCl 3 (d) K 2 O (e) Li 3 N (f) KF. In the Lewis structures listed here, M and X represent various elements in the third period of the periodic table.
Lewis Dot Structures/Diagrams. LEWIS DOT STRUCTURES/DIAGRAMS . Lewis Dot Structure/Diagram for an ATOM: The Electron Configuration for a Nitrogen atom is [He] 2s2 2p3 The Orbital Diagram for N is . Thus, the Valence Shell of N is 2s2 2p3 with a total of 5 Valence Electrons ( = sum of exponents in the Valence Shell). As easier way to determine the number of Valence Electrons is to look at the ...
MOLECULE LEWIS DIAGRAMS. A Lewis diagram depicts a mmolecule using an element symbol to represent the nucleus and core electrons of each atom. Valence electrons are represented by lines for electron pair bonds and dots for unbonded electrons. The following procedure can be followed to derive Lewis diagrams for most molecules. 1.
To change the symbol of an atom, double-click on the atom and enter the letter of the new atom. Show the formal charge of the atom. FREE Expert Solution Group Number = # of valence electrons Aluminum (Al) → Group 3A valence electrons = 3 e- Surround aluminum with 3 dots (electrons). Lewis dot structure for Al: 88% (172 ratings)
Lewis Dot Diagrams… Gilbert Lewis used a different model than Bohr, and he only showed the valence e- in it. His model is called the . Lewis dot structure . He put dots around the symbols so that we can . see. just the . valence electrons . for the elements (so we can easily see which e- are going to react)
A step-by-step explanation of how to draw the AlN Lewis Dot Structure.For AlN we have an ionic compound and we need to take that into account when we draw th...
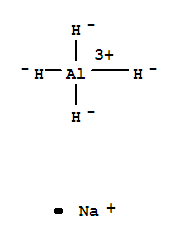
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. charge …. View the full answer. Transcribed image text: Write the Lewis structure for Al_2O_3. Draw the Lewis dot structure for Al_2O_3. Include all lone pairs of electrons.
What is the Lewis Dot Diagram for Aluminum? Aluminum is in Group 13, will have 3 valence electrons, you'll put three "dots" or valance electrons around the element symbol (Al). 1 doublet and one ...
A step-by-step explanation of how to draw the Lewis dot structure for Al (Aluminum). I show you where Aluminum is on the periodic table and how to determine...
Aluminium is exceptional to the octet and it is also called octet deficient. But in the case of the AlCl3 lewis diagram, the Aluminium central atom get's a formal charge equal to zero when it has 6 electrons around. But when an aluminium central atom is distributed with 8 electrons it gets an uneven formal charge.
Here are a number of highest rated Al2o3 Lewis Dot Structure pictures upon internet. We identified it from well-behaved source. Its submitted by presidency in the best field. We recognize this nice of Al2o3 Lewis Dot Structure graphic could possibly be the most trending subject in imitation of we portion it in google pro or facebook.
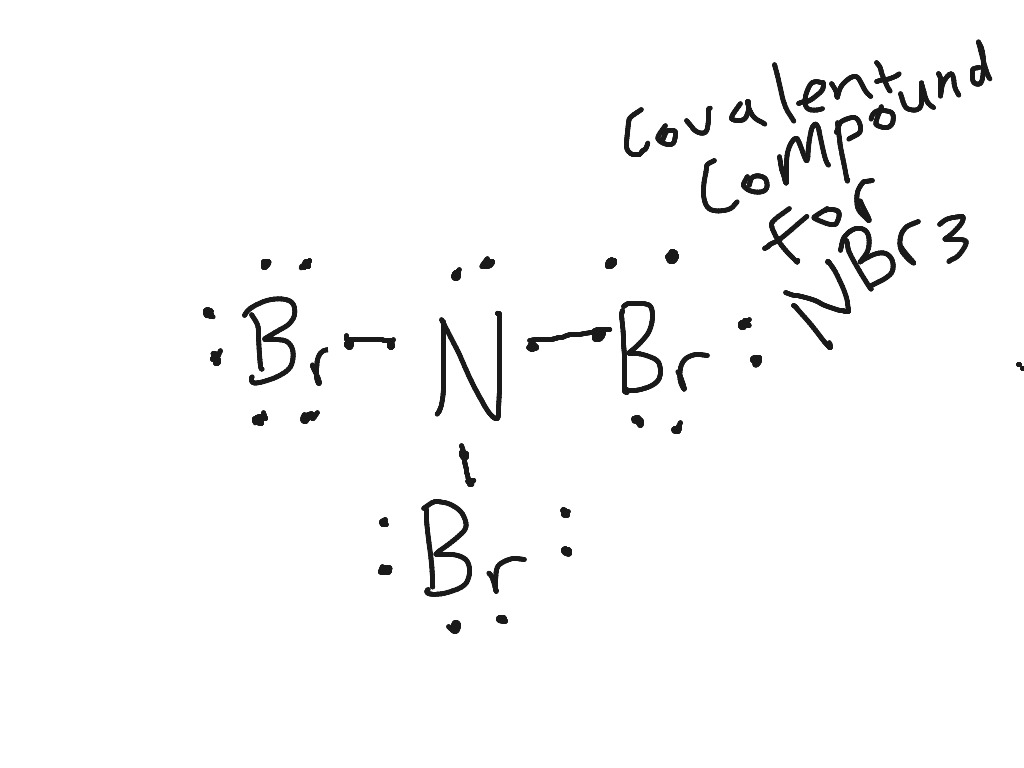







![Answered: çš„[A]](https://prod-qna-question-images.s3.amazonaws.com/qna-images/question/11ab15b2-4b3e-4a6d-a682-972308bf19c4/74d33c47-b954-429e-924f-ec76f7fbb1e9/5vkaezg.jpeg)







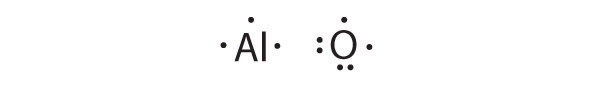



/Lewis-dot-58f78f405f9b581d5938e617.jpg)





0 Response to "40 lewis dot diagram for al"
Post a Comment