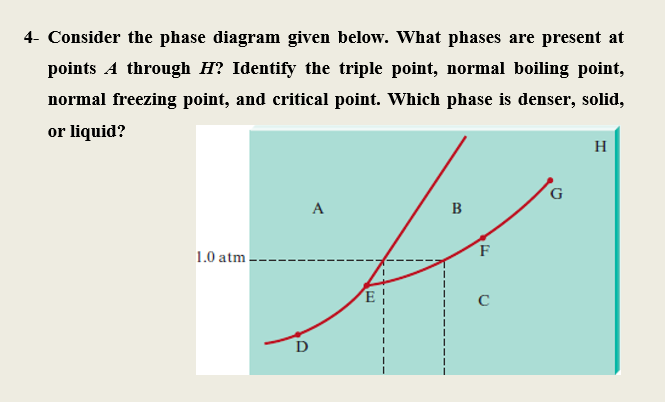
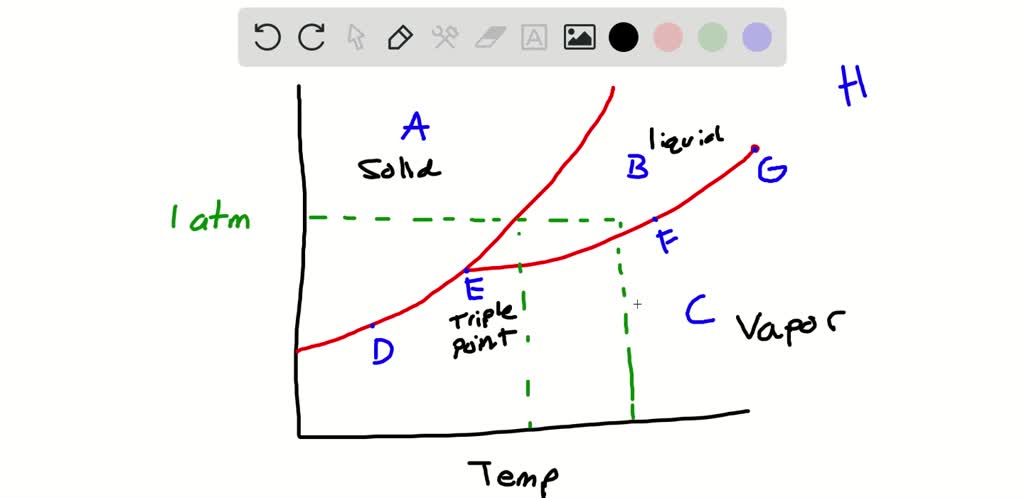
40 examine the following phase diagram and determine what phase(s) exists at point a.
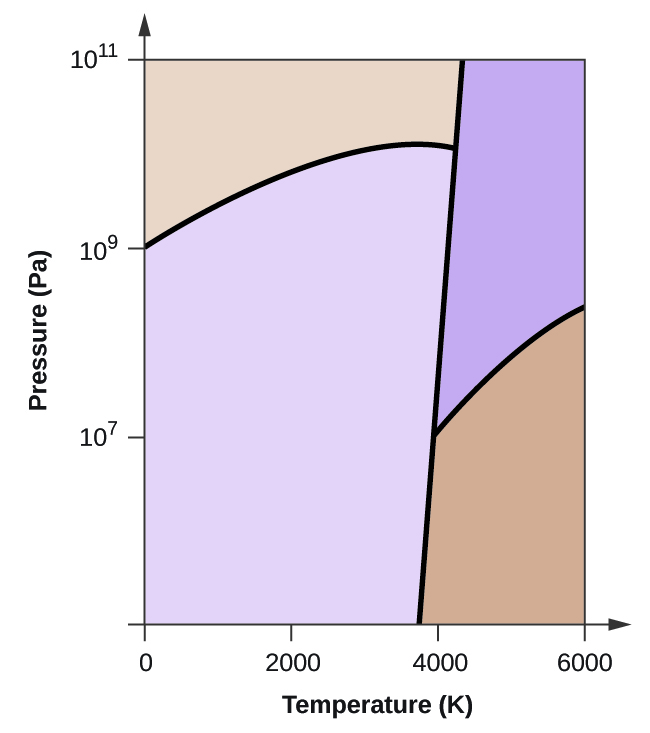
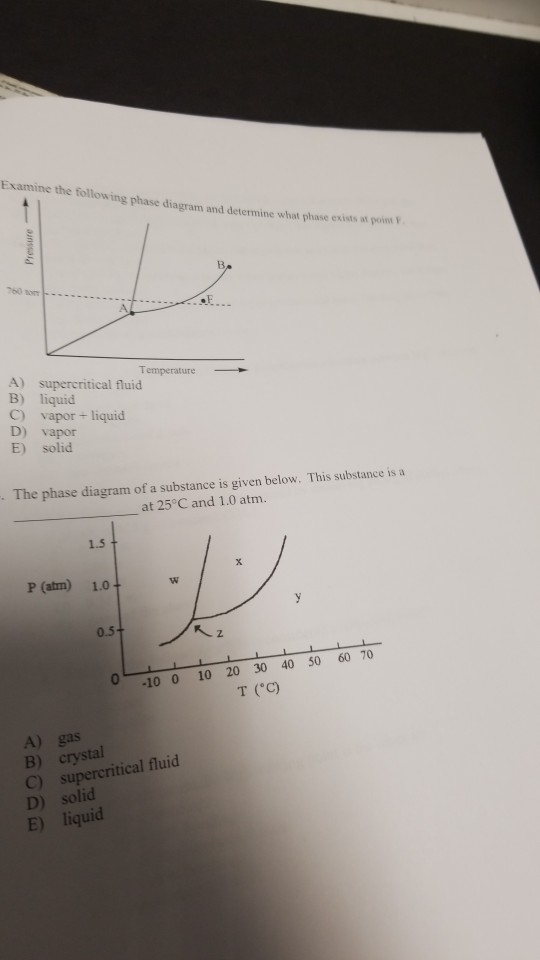
The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ... Examine the following phase diagram and determine what phase exists at point F. Pressure В. 760 torr Temperature a) vapor + liquid b) vapor c) liquid d) solid e) supercritical fluid Question : 13. This problem has been solved!
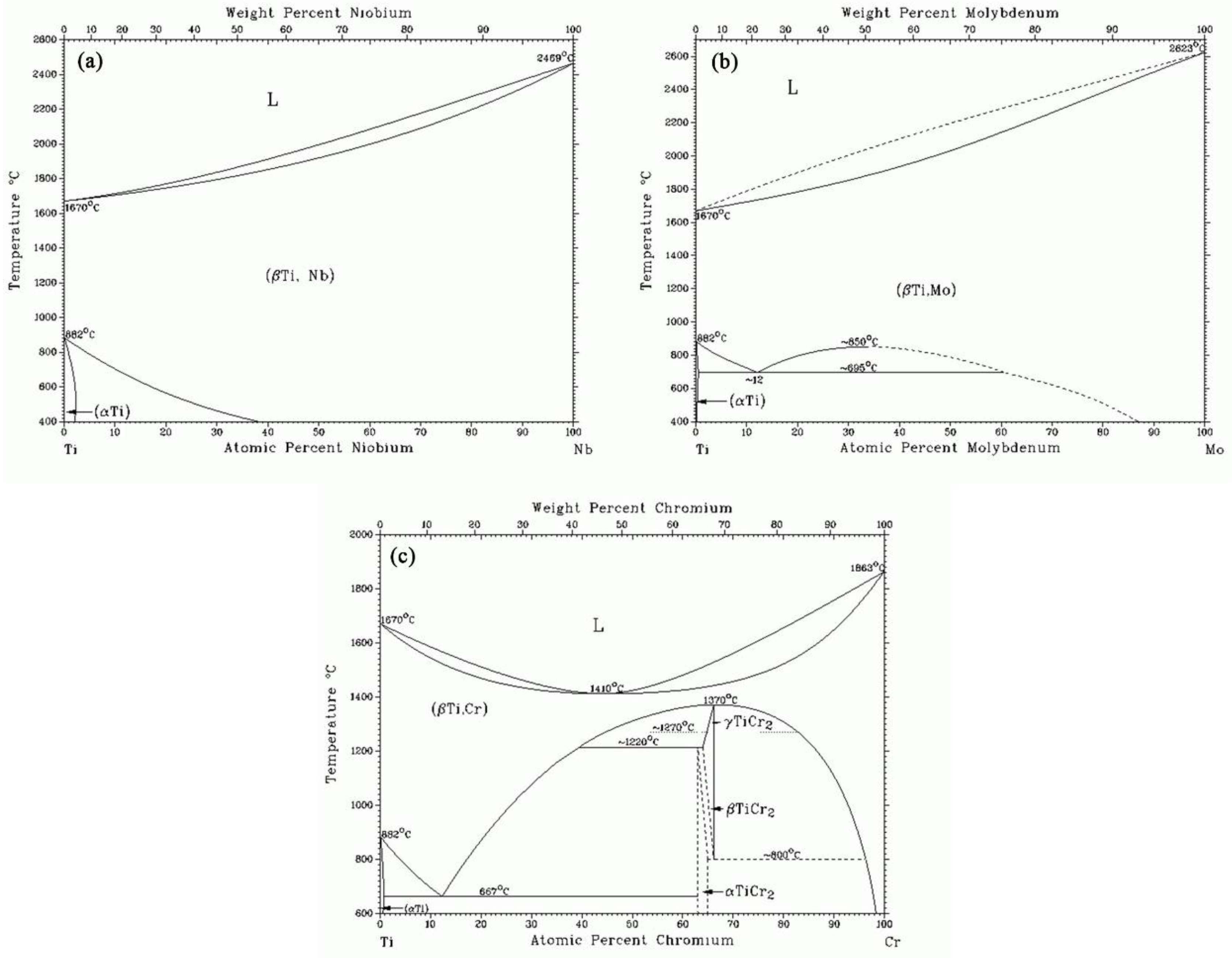
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Examine the following phase diagram and determine what phase(s) exists at point a.
Use phase diagrams to identify stable phases at given temperatures and pressures, and to describe phase transitions resulting from changes in these properties. In the previous module, the variation of a liquid’s equilibrium vapor pressure with temperature was described. Considering the definition of boiling point, plots of vapor pressure ... Examine the following phase diagram and determine what phase exists at point F. A)Vapor + Liquid B)Vapor C)Liquid D)Solid E)Supercritical fluid. Categories Questions. Leave a Reply Cancel reply. Your email address will not be published. Comment. Name. This problem asks that we determine the phase mass fractions for the alloys and temperatures in Problem 9.8. (a) From Problem 9.8a, ε and η phases are present for a 90 wt% Zn-10 wt% Cu alloy at 400 °C, as represented in the portion of the Cu-Zn phase diagram shown below (at point A).
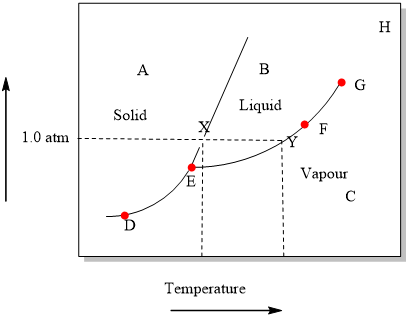
Examine the following phase diagram and determine what phase(s) exists at point a.. PHASE DIAGRAMS: Phase diagrams are another tool that we can use to determine the type of equilibration process and the equilibrium solution. In a phase diagram we graph y(t+1) as a function of y(t). We use a line of slope +1 which passes through the origin to help us see how the time path will evolve. The slope of the phase line A phase diagram is a graph of the physical state of a substance (solid, liquid or gas) and the temperature and pressure of the substance. Phase diagrams are unique to every different substance ... The diagram describes the suitable conditions for two or more phases to exist in equilibrium. For example, the water phase diagram describes a point (triple point) where water can coexist in three different phases at the same time. This happens at just above the freezing temperature (0.01°C) and 0.006 atm. Using the Diagrams. There are four major uses of alloy phase diagrams: Answer: gas phase Hi please have a look at the f… View the full answer Transcribed image text : Examine the fallowing phase diagram and determine what phase exists al poi tom Temperature
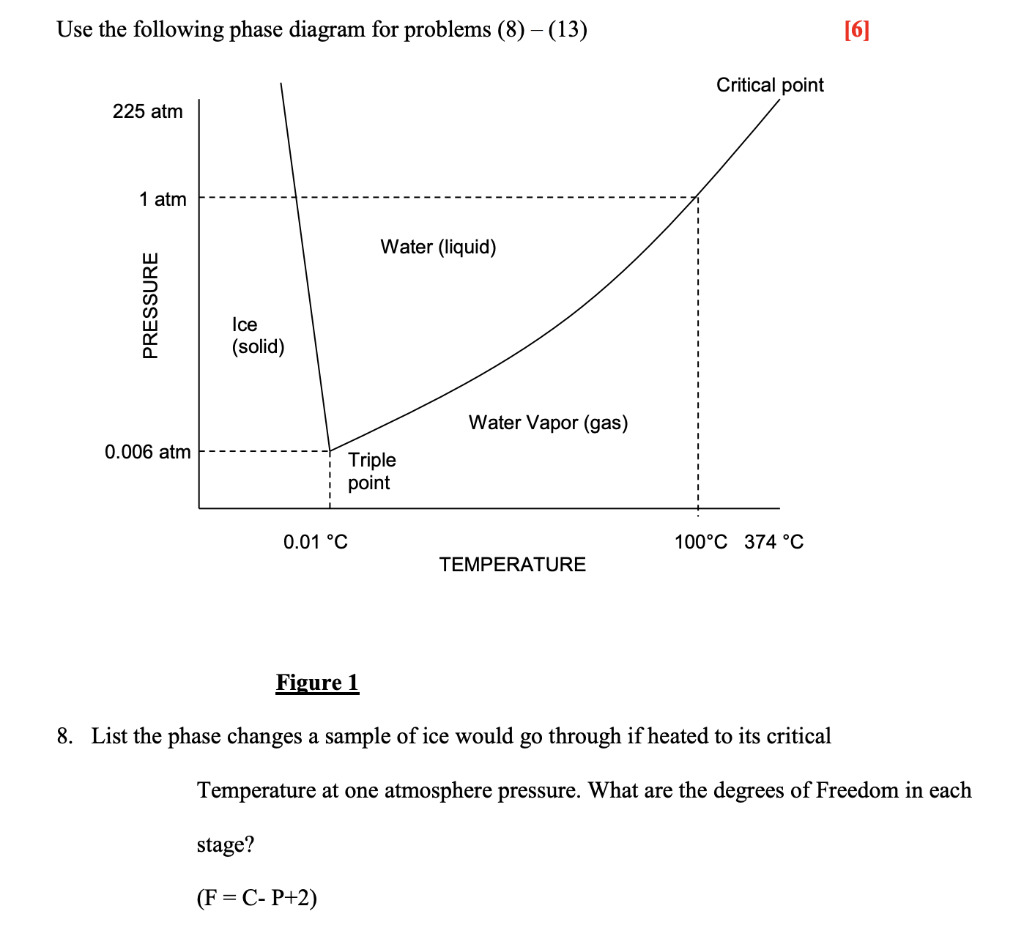
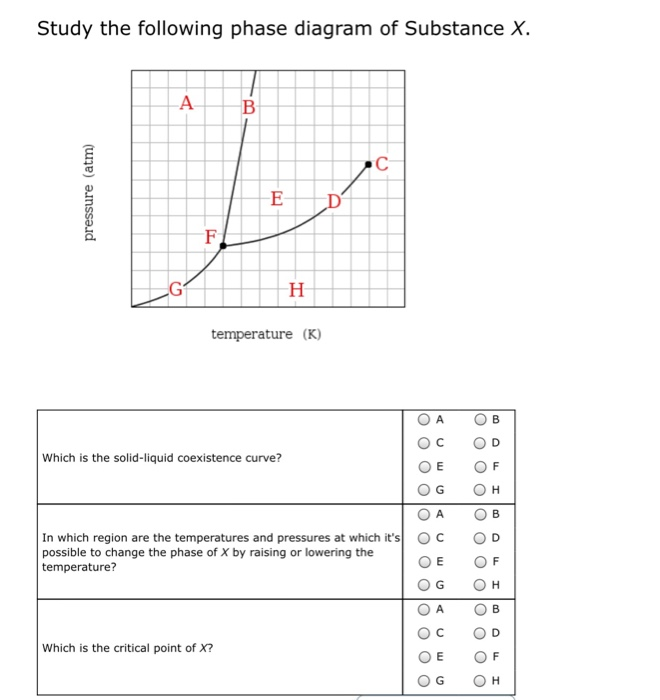
Phase diagram and "degrees of freedom" A phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically-distinct phases; or to show what phases are present in the material system at various T, p, and compositions • "equilibrium" is important: phase diagrams are determined by using slow cooling A typical phase diagram for a pure substance is shown in Figure 1. ... At pressures lower than the triple point, water cannot exist as a liquid, ... Examine the following phase diagram and determine what phase exists at point C. 1. gas and liquid 2. gas 3. liquid 4. solid 5. supercritical fluid; Question: Examine the following phase diagram and determine what phase exists at point C. 1. gas and liquid 2. gas 3. liquid 4. solid 5. supercritical fluid Examine the following phase diagram and identify the feature : 1379199 11. Examine the following phase diagram and identify the feature represented by point A. A) melting point D) sublimation point. B) critical point E) boiling point ...
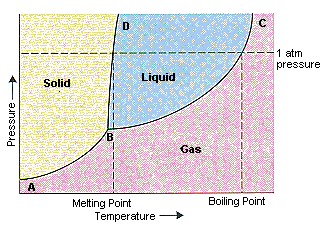
The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point ... These lines are known as phase boundaries. At a point on a phase boundary, the substance can be in either one or the other phases that appear at either side of the boundary. These phases exist in equilibrium with one another. There are two points of interest on a phase diagram. Point D is the point where all three phases meet. Chemistry questions and answers. Examine the following phase diagram and determine what phase exists at point 760 som Temperature A) supercritical fluid B) liquid C) vapor+liquid D) vapor E) solid The phase diagram of a substance i s given below. This substance is a at 25°C and 1.0 atm. 1.5 P (atm) 1.0 0.5T -10 0 10 20 30 40 50 60 70 T ('C) A) gas B) crystal C) supercritical fluid D) solid. Examine the following phase diagram and determine what phase exists at point F Vapor When the electron cloud of a molecule is easily distorted, the molecule has a high _____________.
• Phase Diagrams Phase diagrams are graphs that give information on the equilibrium temperature and pressure for a particular compound. The equilibria occur for the solid- liquid plateau, liquid-vapor plateau and solid-vapor plateau. In this experiment, the phase diagram is shown for the solid-liquid equilibrium point, and varies from 100% ...
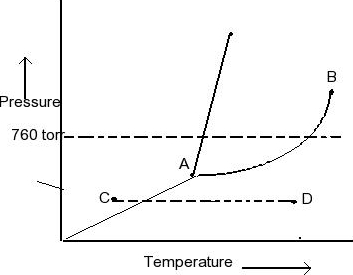
Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. SEE QUESTION 10 Image A) Bo(s) has a lower density than Bo(l). B) The triple point for Bo is at a higher temperature than the melting point for Bo. C) Bo changes from a solid to a liquid as one follows the line from C to D.
Examine the following phase diagram and identify the feature represented by point B. A) Melting point. B) Triple point. C) Critical point. D) Sublimation point. E) Boiling point. Learn this topic by watching Phase Diagrams Concept Videos.
This problem asks that we determine the phase mass fractions for the alloys and temperatures in Problem 9.8. (a) From Problem 9.8a, ε and η phases are present for a 90 wt% Zn-10 wt% Cu alloy at 400 °C, as represented in the portion of the Cu-Zn phase diagram shown below (at point A).
Examine the following phase diagram and determine what phase exists at point F. A)Vapor + Liquid B)Vapor C)Liquid D)Solid E)Supercritical fluid. Categories Questions. Leave a Reply Cancel reply. Your email address will not be published. Comment. Name.
Use phase diagrams to identify stable phases at given temperatures and pressures, and to describe phase transitions resulting from changes in these properties. In the previous module, the variation of a liquid’s equilibrium vapor pressure with temperature was described. Considering the definition of boiling point, plots of vapor pressure ...

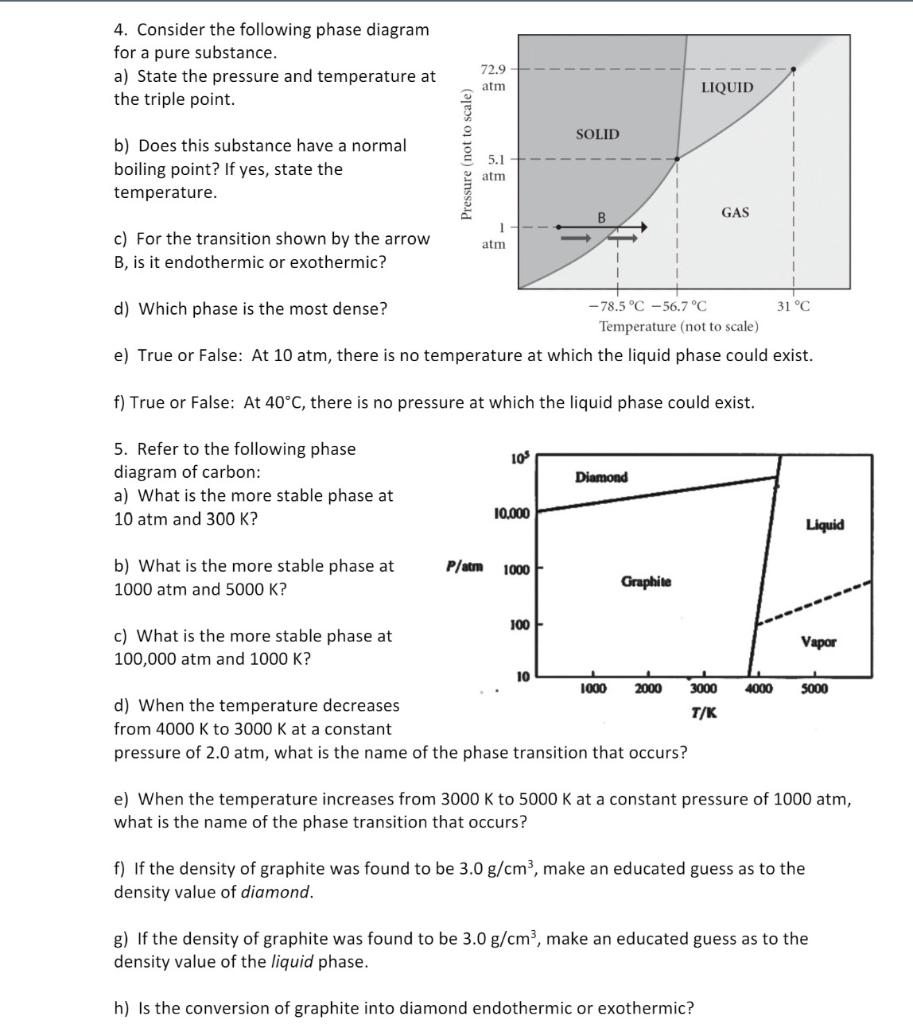
Consider the phase diagram shown. Choose the statement below that is TRUE. A) The triple point of this substance occurs at a temperature of 31°C.B) At 10 atm of pressure, there is no temperature


















0 Response to "40 examine the following phase diagram and determine what phase(s) exists at point a."
Post a Comment