39 hcn molecular orbital diagram
A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge.. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.. In the kinetic theory of gases, the term molecule is … VSEPR Theory Questions and Answers. Get help with your VSEPR theory homework. Access the answers to hundreds of VSEPR theory questions that are explained in a way that's easy for you to understand.
RE: a thank you to entice the C-N molecular orbital diagram for the sigma bond in HCN? Label each and every molecular orbital with its call. Create a molecular orbital diagram of the linear BeF2 molecule. For Be use a basis set that consists of the 2s, 2px, 2py, 2pz atomic orbitals. Unlike in question 4.
Hcn molecular orbital diagram
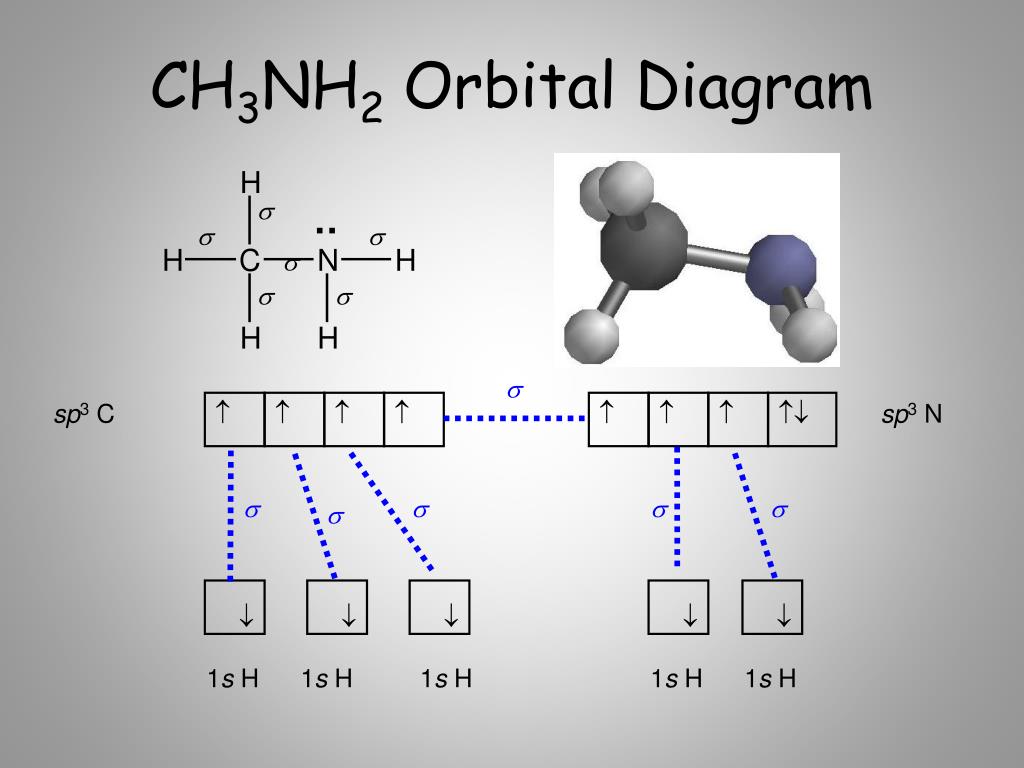
May 17, 2018 · Hydrogen, the exception, needs only two electrons to fill its outer shell. To construct a Lewis diagram, you have to start with a central atom around which all the other atoms congregate. The central atom is the one with the lowest electronegativity, and you can compare electronegativity by looking at the periodic table. F HCN 1 + 4 + 5 =10 ... HCN. 44. 2. F F (MO Theory). F F F (Molecular Orbital Theory) ... (Molecular Orbital Diagram). MO AO. 1sA + 1sB. Bonding. 21) The orbital hybridization on the carbon atom in HCN is. A) sp. B) sp2. C) sp3. D) none of the above. 22) What orbital hybridization is expected for the central atom in a molecule with a trigonal planar geometry?
Hcn molecular orbital diagram. Wong* a Author affiliations Abstract Large-amplitude molecular motions which occur during isomerization can... A natural bonding orbital analysis along the isomerization path further demonstrates that hyperfine... hyperfine structure can be used as a diagnostic tool for characterizing localized HCN and HNC vibrational states.Publishing Journals Books Databases Search Advanced Log in / register About Cited by Related Buy this article... Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 00055) AU Orbital Period 3.52472 (± 2.82e-05) day + Eccentricity 0.0082 ( -0.0082 +0.0078 ) + ω 43.8 (± 68.0) deg + T peri 2452968.399 JD Radius 1.38 ( -0.018... HCN Measurements Detected 2021 - GIACCOBE P. Measurements Detected 2019 - GANDHI S. Measurements Detected 2017 - MACDONALD R. & MADHUSUDHAB N. Measurements Detected... Home All Catalogs Diagrams Bibliography Research Meetings Other Sites VO © 1995-2018 Contact us exoplanet... A) The total number of molecular orbitals formed doesn't always equal the number of atomic orbitals in the set. B) A bond order of 0 represents a stable chemical bond. C) When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular ...
Jan 15, 2022 · In the case of HCN, let us look at how the atomic orbitals fuse to make molecular orbitals. Electronic configuration of C is 2s2 2p2, electronic configuration of H is 1s1, and electronic configuration of N is 2s2 2p3. Here, one sp orbital of C fuses with 1s orbital of H. And the other sp orbital of C fuses with one of the p orbitals of Nitrogen. We have explored the potential energy surface for the dissociation of the pyridazine molecular ion using G3 model calculations. The pathways have been obtained for the formation of five possible C 4 H +∙ 4 isomers by the loss of N 2 and the consecutive H ∙ loss. It is predicted that the methylenecyclopropene radical cation... the bonding between the carban and the nitrogen in hydrogen cyanide or hydrocyanic acid is a triple bond, hence the hybrid orbital is sp, due to the linear geometry of the molecule Download scientific diagram | HOMO and LUMO orbitals of HCN molecule on the Mg and Ca-doped BeONTs from publication: Boosting BeONT Reactivity with HCN by Calcium and Magnesium Doping: A DFT ...
Hydrogen cyanide is a one- carbon compound consisting of a methine group triple bonded to a nitrogen atom It has a role as a human metabolite, an Escherichia coli metabolite and a poison. It is a hydracid and a one- carbon compound. It is a conjugate acid of a cyanide. It is a tautomer of a hydrogen isocyanide. Describe HCN molecular bond by using Valence Bond Theory. Because px orbital of C and N will form sigma bond, this leaves with two N atom p-orbitals which form two mutually perpendicular pi bonds to the two atomic p orbitals on the C atom. HCN thus has one single and one triple bond. Click to see full answer. A linear molecule with bond distances correct to 1% of the microwave spectroscopy values is obtained. Walsh's orbital-energy diagrams are found to be almost ... Answer (1 of 7): Summing up the number of σ -bond formed by the desired atom (here N) and the number of lone pair on it we can easily know the hybridization of it. If the sum is 2 →hybridization−sp If the sum is 3 →hybridization−sp2 If the sum is 4 →hybridization−sp3 If the sum is 5 →hybrid...
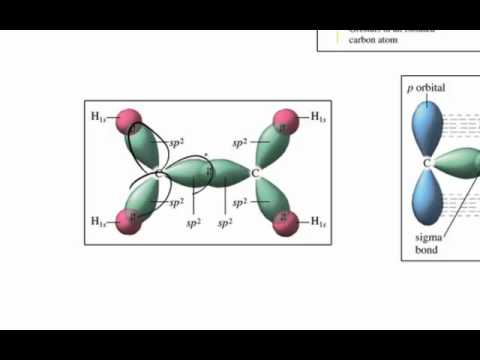
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
The dipole moment in a nitrile group (computed for HCN) is 2.89 debyes, while that of CO is 0.14 debyes. The direction of the dipole moment in both molecules is ...
Mar 25, 2021 · Generally, molecules with linear molecular geometry have sp hybridization as the central atom forms bonds with two atoms only. Number of sigma bonds in the molecule. Another way to find out the hybridization of the molecule is to look at the number of sigma bonds formed. In HCN, there are two sigma bonds, C-H and C-N. The number of sigma bonds is equal to the number of hybrid orbitals formed.
Further Chem: Drawing out the molecular orbital diagrams may help as it would show which electrons enter which sub shell and that would show you if it is entering... If you place CH2Cl2 and CHCl3 on a cartesian diagram so that the overall dipole would point to a value of -Y (straight down, traditionally), then the C-Cl bonds...
The sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to one of the 2p atomic orbitals. The combination of these atomic orbitals creates three new hybrid orbitals equal in energy-level. Which of the following will show sp2 hybridization? Sulphur in SO2, is sp2 ...
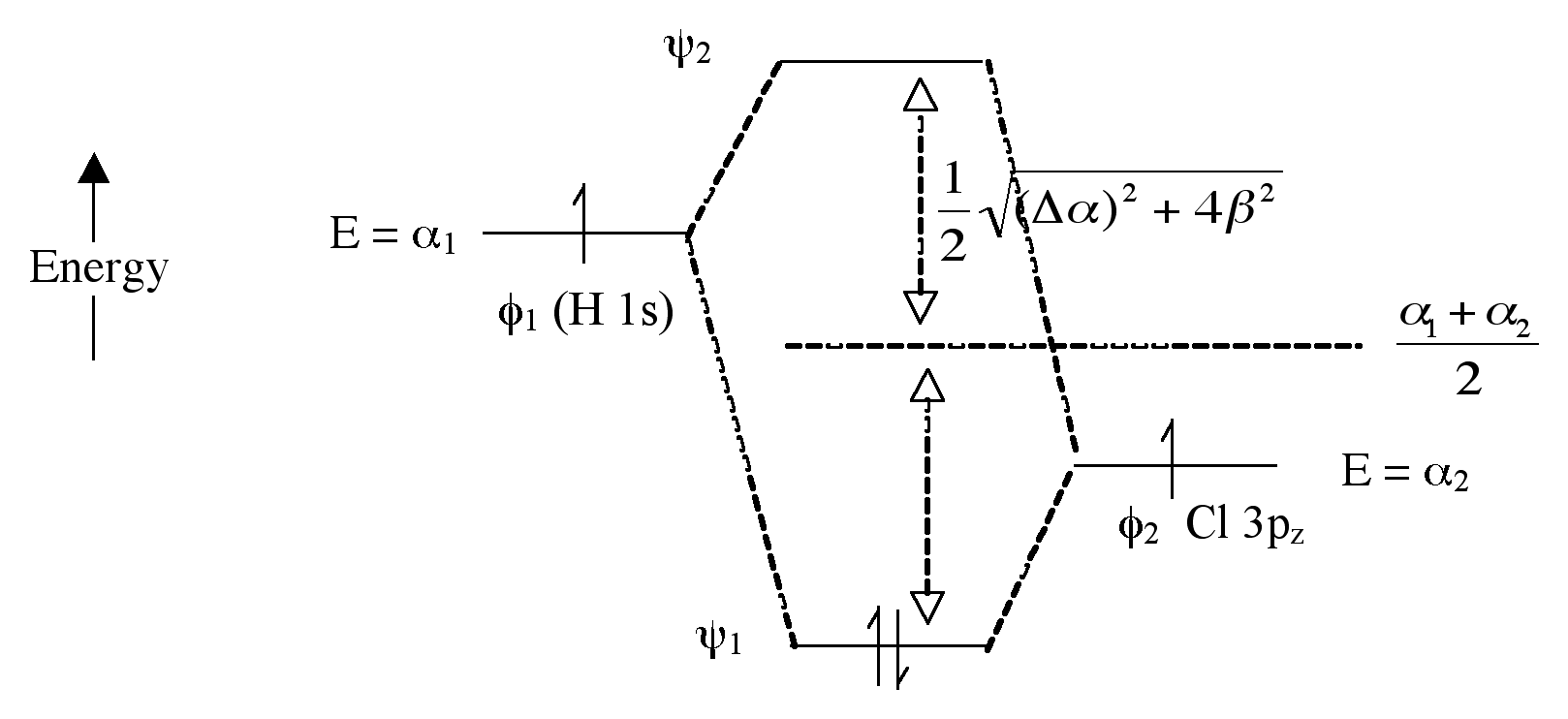
the diagram: carbon, nitrogen, and oxygen. Notes Electron-dot structures of polyatomic molecules Keywords... cyanide, HCN. Notes For Worked Example 7.4 Keywords hydrogen cyanide, structure Title Incomplete Structure... Notes Structure of nitrous oxide Keywords nitrous oxide Title Molecular shapes Caption Three-dimensional... Title Molecular Orbital Diagram Caption Figure 7.15 A molecular orbital diagram for the H2 molecule. The two...
This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl2 molecule. Each of the two sp hybrid orbitals holds one electron ...
ocr.org.uk/alevelchemistrya Oxford Cambridge and RSA CHEMISTRY A H432 For first assessment in 2017 A LEVEL Version 2.6 (December 2020) Specification Qualification Accredited Registered office: The Triangle Building Shaftesbury Road Cambridge CB2 8EA OCR is an exempt charity. Disclaimer Specifications are updated over time....
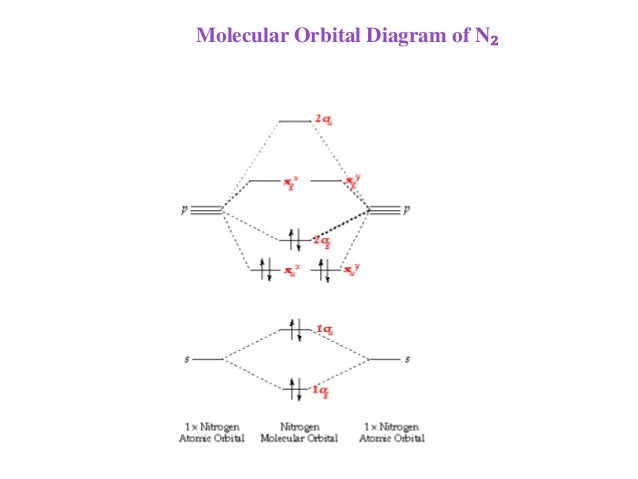
2. The bond order of a homonuclear diatomic molecule can be decreased by. removing electrons from a bonding MO or adding electrons to an antibonding MO. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) N2^2+. B) B2^2+. C) B2^2-. D) C2^2-. E) B2.
CH 10 Bonding & Molecular Structure: Orbital Hybridization & Molecular Orbitals. a) MO theory predicts that electrons are delocalized over the molecule. b) VB theory predicts that oxygen is paramagnetic, MO theory does not. c) VB theory describes a molecular bond as the overlap between two atomic orbitals.
There are two sigma bonds in HCN: C-H and C-N. The number of sigma bonds created equals the number of hybrid orbitals. As a result, the hybridization for the HCN molecule is sp hybridization. Molecular Geometry. The liner molecular geometry of hydrogen cyanide has bond angles of 180 degrees.
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the chemical formula HCN. It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F).HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals.
Transcribed image text: below are the four molecular orbitals of butadiene. Which two orbitals are occupied with electrons ? g6 & 88 & gf & g88 8888 D c B A A. Cand D B. A and c C. A and D D. Bandc Select the product of this reaction: 1 HCN NaCl 2.
species (HCN, H CO, CO, CS, CH OH, and HNC) in both comets. Multiline observations of the CHOH = 5–4 series allow us to estimate the rotational temperature using the rotation diagram technique. We derive rotational... symmetric molecular excitation code that includes collisions between neutrals and electrons. The effects of...
1 day ago · Molecular Orbital Diagram. Molecular Orbital Theory is slightly different from VBT and orbital hybridization. Here, AOs from different atoms inside the molecule can come together to form molecular orbitals or MOs. Therefore, valence electrons are shared inside the molecule. The electronic configuration of both C and N are as follows: Carbon ...
Molecular Geometry. Hydrogen cyanide has linear molecular geometry with bond angles of 180 degrees. As hydrogen and nitrogen tend to be far from each other, HCN forms a linear shape. It is slightly polar as nitrogen tries to pull the electrons to itself due to its electronegative value.
& Astrophys., 633, A48 Observability of molecular species in a nitrogen dominated atmosphere for 55 Cancri e Can Planets Exist in the Habitable Zone of 55~Cancri? Multi-season optical modulation phased with the orbit of the super-Earth 55 Cnc e SULIS S., DRAGOMIR D., LENDL M.,BOURRIER V., DEMORY B. et al. Astron. & Astrophys....
Fill in the molecular orbitals in the molecular orbital diagram for CO. One 2 s and three 2 p orbitals from carbon and one 2 s and three 2 p orbitals combine to form eight molecular orbitals in C O. The molecular orbitals in order of increasing energy are one sigma 2 s, one sigma 2 s star, two pi 2 p, one sigma 2 p, two pi 2 p star, and one ...
a) The nerve gas Sarin, which was released in a Tokyo subway station in 1996, has a molecular formula of C4H10PO2F. Determine the composition (percent by mass) of... a) A compound containing 79.37% C, 8.88% H, and 11.75% O was found to have a molecular mass of approximately 270 g. For this compound, determine the empirical...
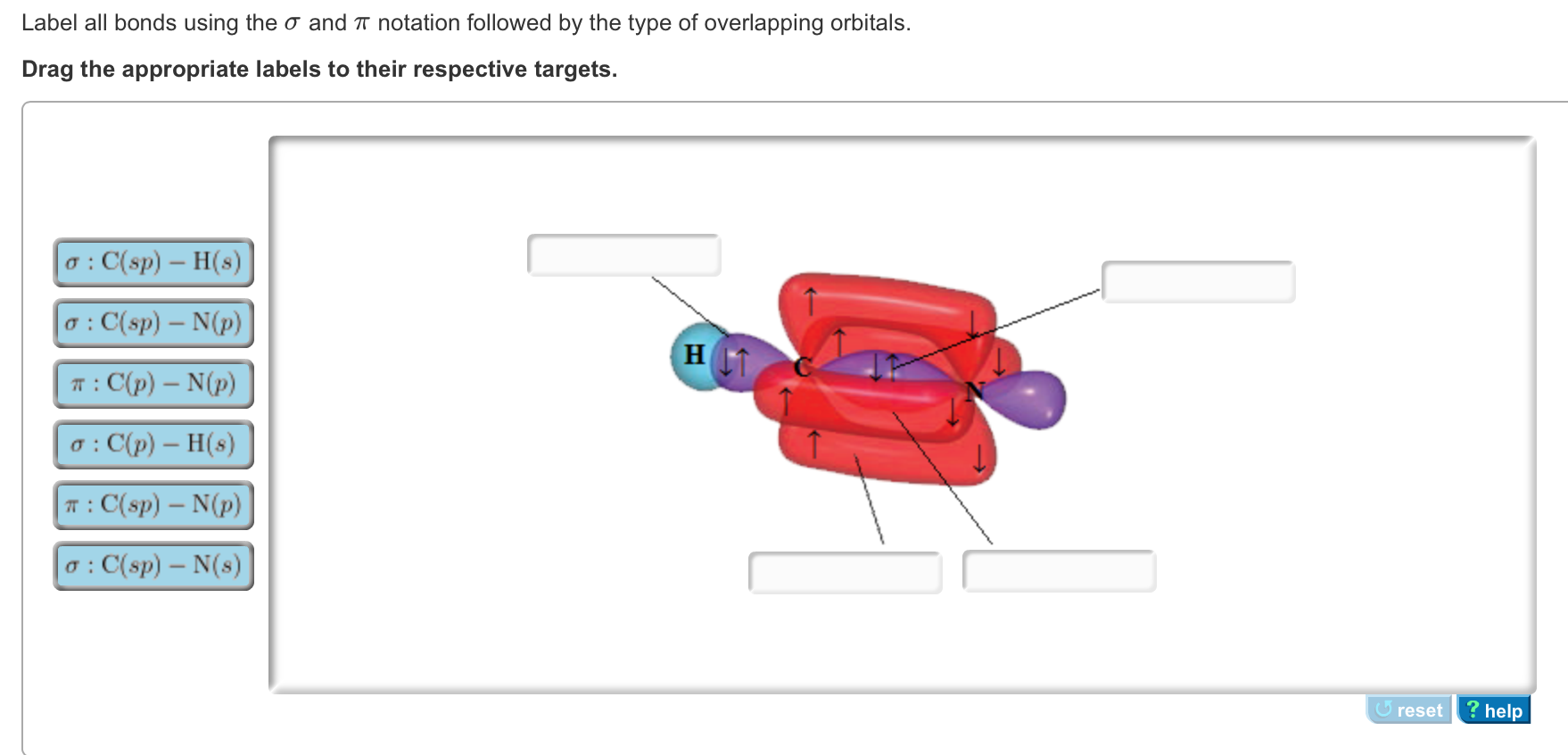
In the HCN molecule, C atom includes sp - hybridized orbital, since it will combine with only two other atoms to form HCN. One of the sp - hybrid orbitals of Carbon atom overlaps with 1 s orbital of H atom, while other sp - hybrid orabital mixes with one of Nitrogen's atom's three atomic p orbitals which were unhybridized.
For the molecule hydrogen cyanide (HCN):a) Draw the filled atomic orbitals of the nitrogen atom.b) Which atomic orbitals are used for hybridization and event...
How to make molecular Orbital diagramhttps://www.youtube.com/watch?v=UYC-ndQ6Lww&t=6s
a molecular jet with a projected length of 150 pc. The launch region is unresolved and lies inside a... A simple model of a molecular jet precessing around an axis close to the plane of the sky can reproduce the... The CO emission is clumpy along the jet and the total molecular mass in the high-velocity ( (60 to 150)) gas lies...
Jul 23, 2021 · Once we know the Lewis structure and Molecular Geometry of any molecule, it is easy to determine its bond angles and polarity. As this molecule has a linear molecular geometry, HCN has bond angles of 180 degrees. HCN Shape. As both Hydrogen and Nitrogen are placed far from each other at bond angles of 180 degrees, it forms a linear shape. HCN Polarity
single diagram. In cases such as these, the electron delocalization described by resonance enhances the stability of the molecules, and compounds composed of such... A molecular orbital description of benzene provides a more satisfying and more general treatment of "aromaticity". We know that benzene has a planar hexagonal...
Use the molecular orbital theory to determine the ground-state electron configuration of F_2 and F_2^{+}. View Answer An element with 37 protons in …
(a) Molecular Orbital Diagram for Single Pancake-Bonded Dimers; (b) Molecular Orbital Diagram for Double Pancake-Bonded Dimers Based on a Triplet Ground State of the Monomer; (c) Molecular Orbital Diagram for Double Pancake-Bonded Dimer Based on a Singlet Diradicaloid Ground State of the Monomer with a Low HOMO–LUMO Gapa... (a) Molecular Orbital Diagram for Single Pancake... Structures of two substituted dithiatriazine (HCN 3 S 2 )...
Molecular orbital theory is also able to explain the presence of Figure \(\ PageIndex{6}\): Molecular Orbital Energy-Level Diagram for HCl. to describe the bonding in the cyanide ion (CN −). mix atomic orbitals on different atoms to get Molecular Orbitals. The resul7ng MO diagram looks like this. CN– (Cyanide ion), NO+ (Nitrosonium ion).
Water is the chemical substance with chemical formula H 2 O; one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Water is a tasteless, odorless liquid at ambient temperature and pressure.Liquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. This can easily be observed in …
May 19, 2019 ... Molecular orbital energy level diagram for HCN. Exercise 10.7.1. Describe the bonding in formaldehyde (H2C=O), a trigonal planar molecule, ...
May 8, 2021 ... A Because HCN is a linear molecule, it is likely that the bonding can be described in terms of sp hybridization at carbon. Because the nitrogen ...
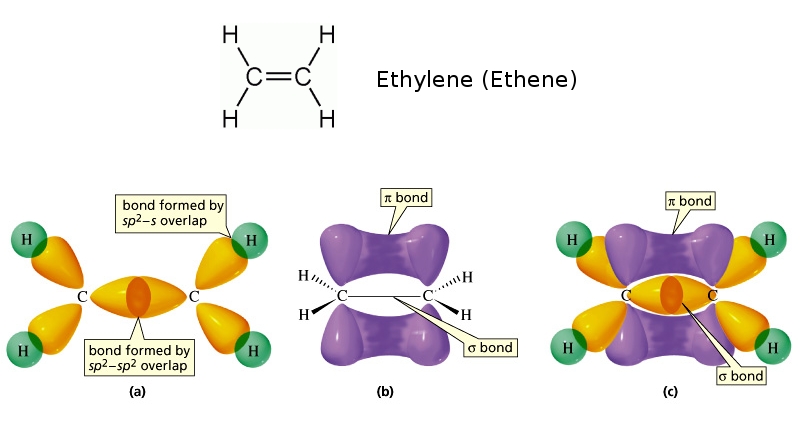
As seen in Table 7.4.1, an average carbon-carbon single bond is 347 kJ/mol, while in a carbon-carbon double bond, the π π bond increases the bond strength by 267 kJ/mol. Adding an additional π π bond causes a further increase of 225 kJ/mol. We can see a similar pattern when we compare other σ σ and π π bonds.
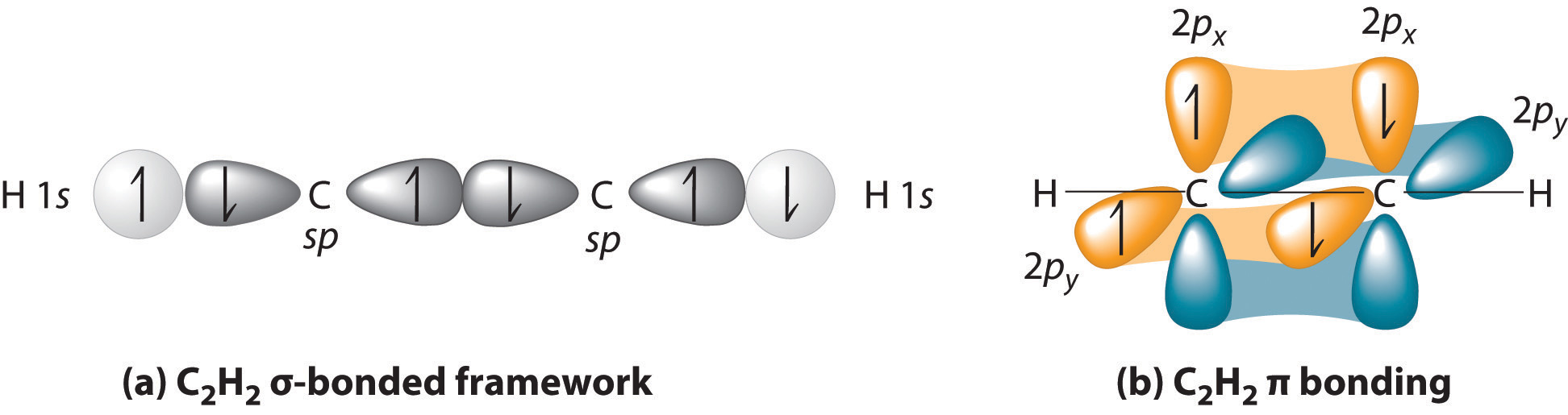
Sigma (σ) bonding molecular orbital - Shared electron density is directly ... (H-CC-H) as a model to draw an MO diagram for hydrogen cyanide (H-CN) and.
Here, s, p, and d are atomic orbitals and z is the bond axis. Now, the covalent chemical bonds which are formed when the atomic orbitals overlap laterally are known as pi ($\pi $) bonds. Usually, there is 1 pi ($\pi $) bond in double bonds and 2 pi ($\pi $) bonds in triple bonds.
bringing you the latest astronomy papers uploaded to astro-ph ... High contrast imaging with Fizeau interferometry: The case of Altair [IMA] Posted on January 19, 2022 by arxiverbot http://arxiv.org/abs/2201.05897 The Large Binocular Telescope (LBT) has two 8.4-m primary mirrors that produce beams that can be combined coherently in a “Fizeau” interferometric mode. In principle, the Fizeau PSF enables the probing of structure at a resolution up to three times better than that of the adaptive-optics-corrected PSF of a single 8.4-m telescope. In this work, we
Previous Chapter Table of Contents Next Chapter Chapter 9 Molecular Geometry and Covalent Bonding Models In... predict molecular geometries. To predict whether a molecule has a dipole moment. The Lewis electron-pair... Groups are positioned around the central atom in a way that produces the molecular structure with the lowest... 33 "Molecular Orbital Energy-Level Diagram for π Bonding in Ethylene... in HCN using a combination of...
Oct 06, 2020 · an infamous small molecule known as hydrogen cyanide is shown draw a molecular orbital diagram. then draw MOs of the HCN molecule in the middle. indicate which MO is LUMO AND HOMO The molecular orbital diagram of NO shown in Figure 10.47 also applies to OF .
Energy level diagram of HCN (a) and the HCN-water complex (b). Molecular orbitals (MOs) marked in red and black are included in the active spaces for RIXS ...
Cyanide Molecular Orbital Diagram. MO Theory: the bonding orbital will be lower in energy, the an7bonding The resul7ng MO diagram looks like this. CN– (Cyanide ion), NO+ (Nitrosonium ion ). The molecular orbital diagram of (if order of molecular orbital is like that in) is as shown below. We must remember that total number of electrons in ...


















0 Response to "39 hcn molecular orbital diagram"
Post a Comment