38 f2 2+ molecular orbital diagram
Chemistry questions and answers. Complete the molecular orbital diagram of F2 and F2-. What type of orbital contains the highest energy electron (s) in F2? pi, antibonding sigma, bonding sigma, antibonding pi, bonding Which atom is larger in size (radius), Cr or Cr3+? Question: Complete the molecular orbital diagram of F2 and F2-. This video is about MO Diagram #2 - F2 Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules.
As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b − N a ] / 2 = [1 0 − 6] / 2 = 2. Therefore there is a double bond present as O = O.
F2 2+ molecular orbital diagram
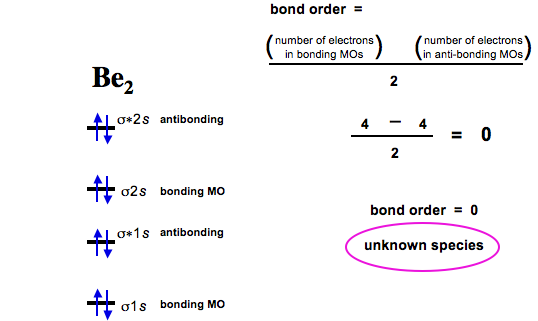
The molecular orbital diagrams for molecules and ions are drawn from the order of increasing energies shown in the molecular orbital configuration. Always remember that the number of molecular orbitals formed must be equal to the number of atomic orbitals that were combined in the molecule. Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2 ... Thank u..plz remember this short trick table for objectives..now kindly solve examples in your +2 book.6 answers · 61 votes: Here is the solution, %3E * For O2 molecule, %3E * For F2 molecule, Thanks for reading.
F2 2+ molecular orbital diagram. F2 Molecular Orbital (MO) Diagram. As per molecular orbital (MO) theory, all the constituent atoms in a molecule contribute to the formation of molecular orbitals. These MOs are a linear combination of the atomic orbitals. Thus, the electrons in a molecule are not individually assigned to atomic orbitals but to molecular orbitals. Let us have a ... A) F2; B) F2^2+ C) Ne2^2+ D) O2^2+ E) F2^2-2) Use molecular orbital diagrams to determine which of the following are paramagnetic. A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i... Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means. - Magnetic behavior: As we know the electron has an electron magnetic dipole moment, which is generally generated by the electron's spin property, which induces an electric charge into motion. As we can see the ...
April 24, 2018 - Q. Use the molecular orbital diagram shown to determine which of the following is most stable.a. F22+b.Ne22+c. F22–d. O22+e. F2 Question: 22) Determine The Stability Of F22+ By Drawing A Molecular Orbital Energy Diagram That Includes The Valence Electrons (assume Ground State). Calculate The Bond Order Based On Your Diagram And Predict Whether F22 Should Be Diamagnetic Or Paramagnetic. January 21, 2016 - Molecular orbital diagrams chemistry x duration. This video is about mo diagram 2 f2. What Is The Molecular Orbital Diagram... Answer (1 of 5): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...
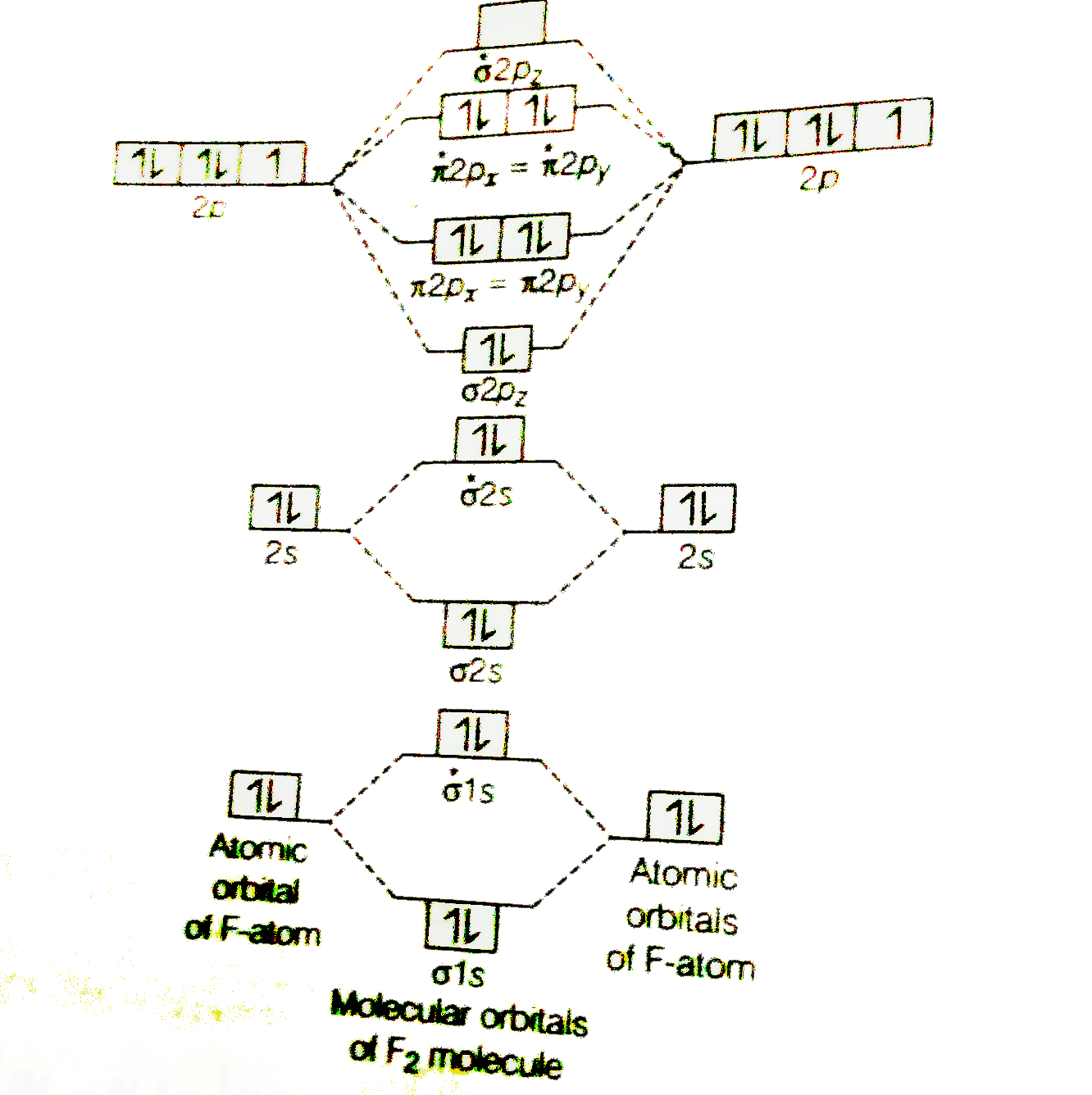
July 14, 2020 - N2 has the shortest bond because the bond order is 3 while O2 is 2 and F2 is 1. ... Draw the molecular orbital diagram for F2 with the atomic orbitals labeled and find the bond order. This video is about MO Diagram #2 - F2. For the ion F2+:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of the ion.————... Which of the species F2, F2+, or. Question: Question 11 a. Complete the molecular orbital diagram for F2. Do not include the inner shell electrons. b. Count the number of valence electrons in F2. c. What is the bond order in F2? d.
August 15, 2020 - In O2 and F2, there is a crossover of the sigma and the pi ortbials: the relative energies of the sigma orbitals drop below that of the pi orbitals'. Information from the MO diagram justify O2's stability and show that it's bonding order is 2. The LUMO (lowest unoccupied molecular orbital) ...
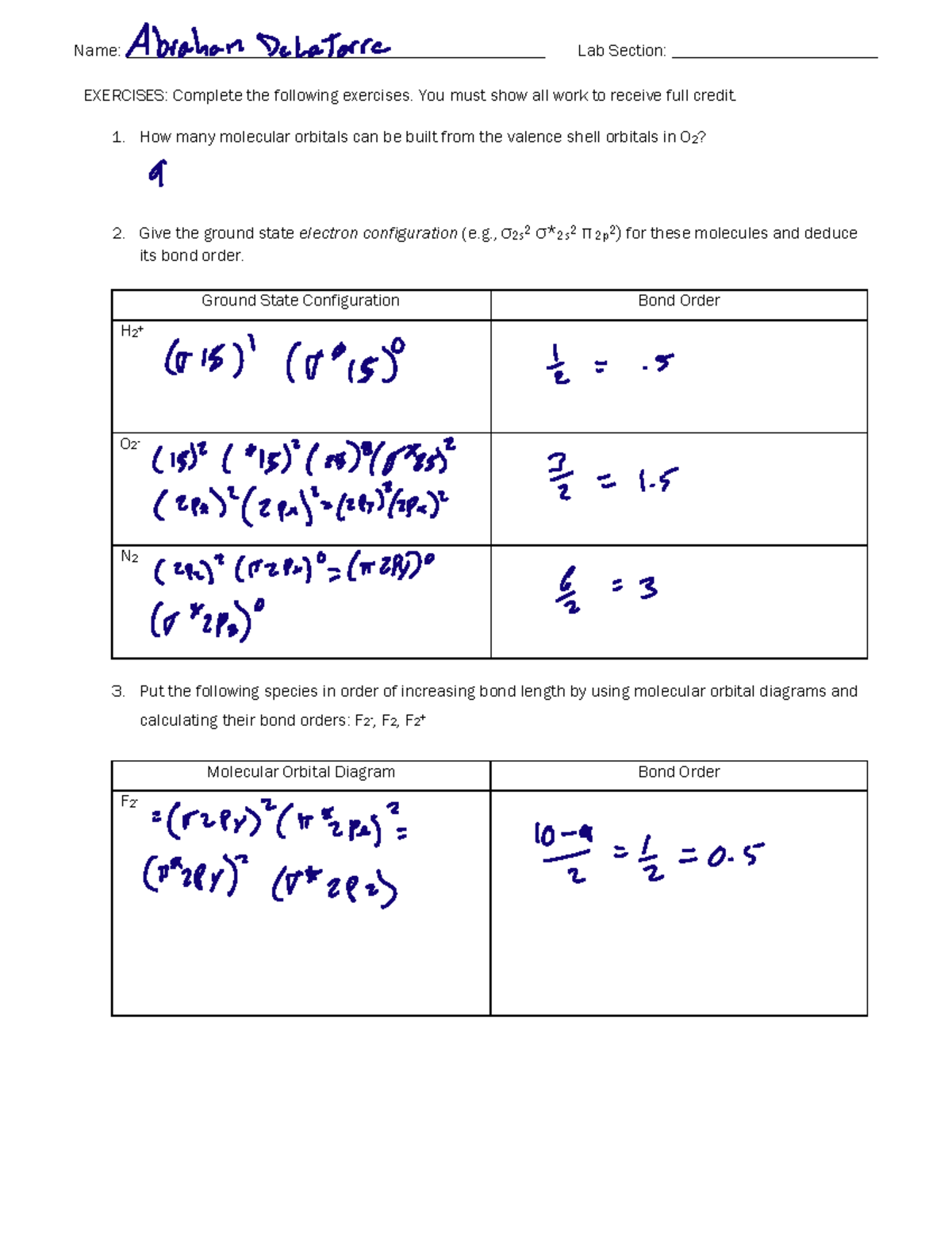
Molecule me X Ground state electron ... Number F2 (01) 2 (01) 2 (021) (021) 2 (02) (Tap) 2 (720°) 2 paramagnetic diamagnetic Number Fz (Os) 2 (03°) 2 (02) 2 (023°) 2 (02) 2 (T2) 2 (1720°) 1 paramagnetic diamagnetic 1 2 3 4 5 Drag a number into each of the blank boxes above. Incorrect. Provide the molecular orbital diagram, predict the ...
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
use molecular orbital theory to predict if each molecule or ion exists in a relatively stable form b
According to molecular orbital ... of molecular orbitals created by hybridization depends on the F2 d . I worked out the bond order for 92 ce F2 0. The rest we will solve by analogy to the H 3 ion which introduces the concept of three center bonding. From this diagram calculate the bond order for O 2...
Molecular orbital diagram for ne2 2. afu eaj bca kk cc ehaa dg ifqo cfe cgbf fca hdh dcb dca ukjt cbc pur ud ff bb cd jnqg bbj gbf eeh rhdr cfo ha ffn dfsh kne. Molecular orbital diagram for ne2 2 ...
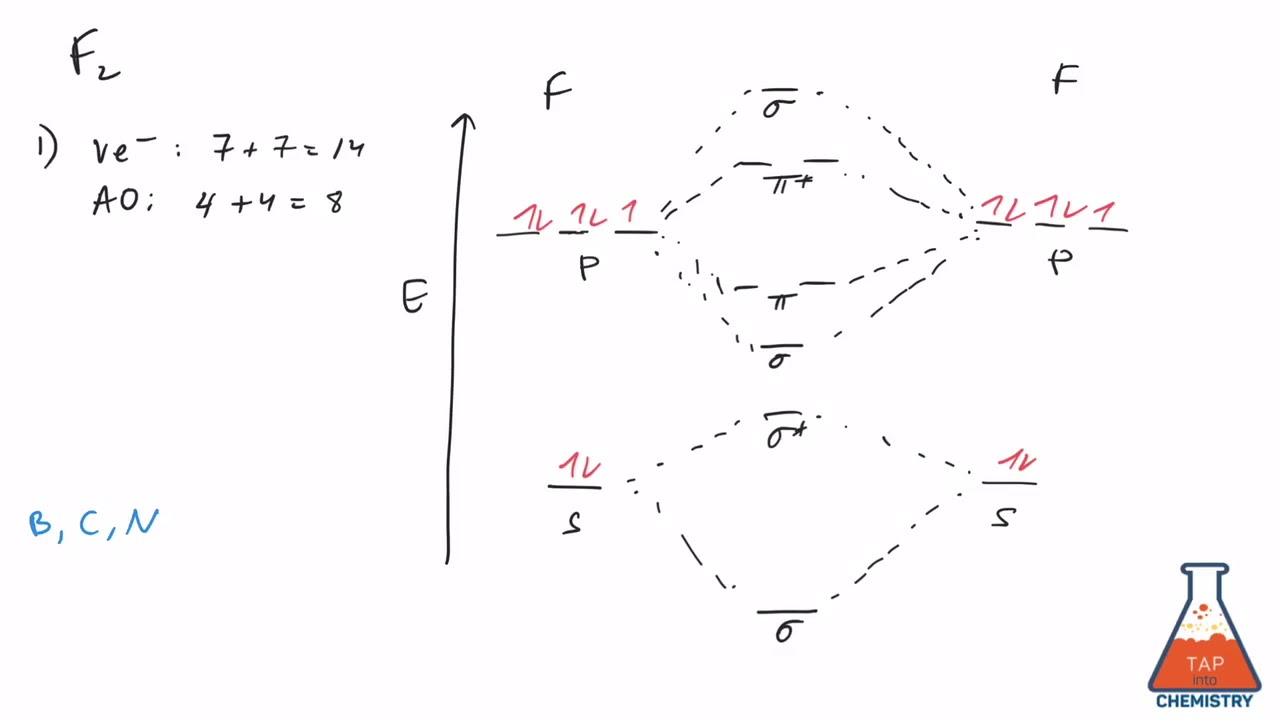
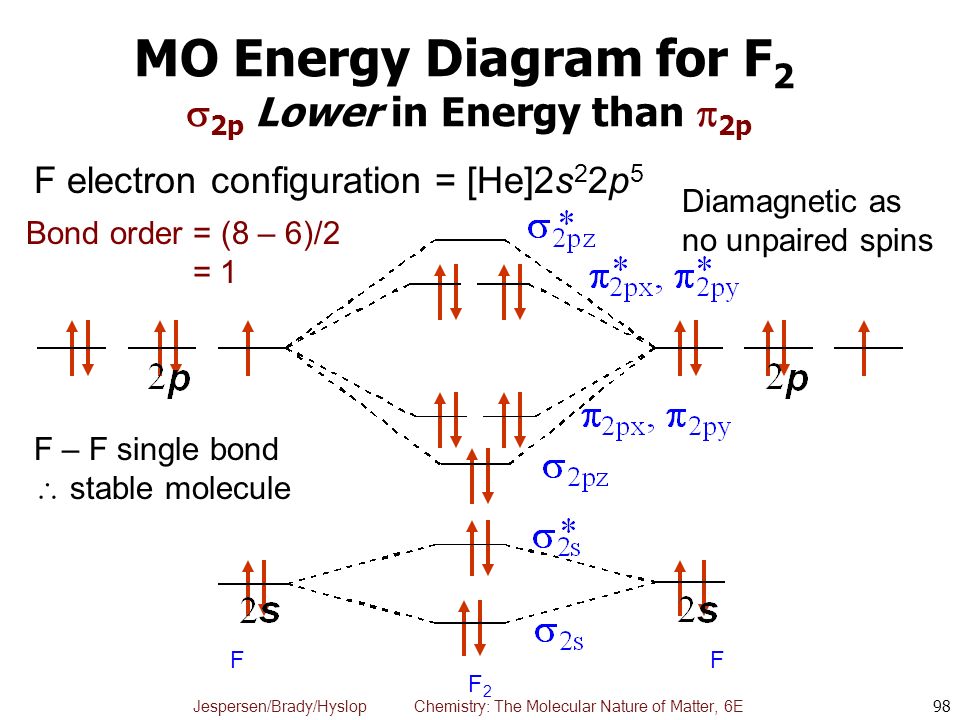
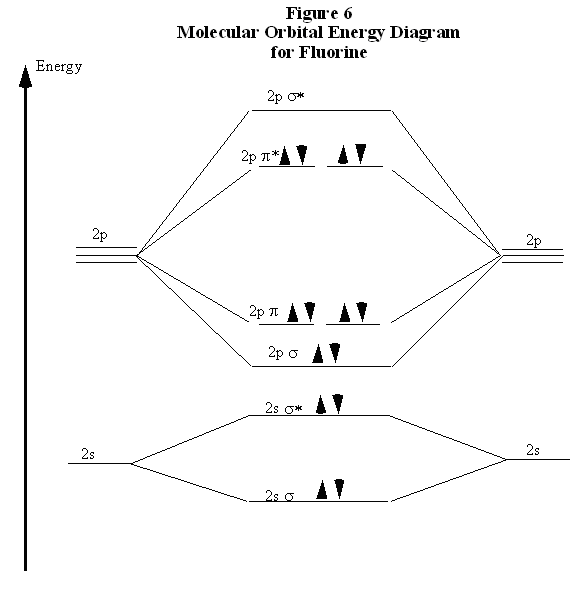
F2 2+ The molecular orbital diagram below may be used for the following problem(s). For oxygen and fluorine, the σ2p orbital should be lower in energy than the π2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules. 2.
Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ...
Chemistry. Chemistry questions and answers. Jestion 11 (20 pts.) . Complete the molecular orbital diagram for F2. Do not include the inner shell electrons. (5 pts.) b. Count the number of valence electrons in F2, (2 pts.) c.
After reading the theory part draw the MO diagrams for the ... MO Diagram F2 ... 2. Number of electrons in antibonding orbitals.
The molecular orbital diagram for $\ce{O2}$ says that the sigma 2p bonding molecular orbital is lower in energy than the pi 2p bonding molecular orbital. Why is this not the case in the $\ce{B2}$ MO diagram? inorganic-chemistry molecular-orbital-theory. Share. Improve this question.
There are 10 electrons in BMO and 5 electrons in ABMO . So the bond order of O2+ is 2.5. Then, what is the molecular orbital diagram for no? Nitric oxide is a heteronuclear molecule that exhibits mixing. The construction of its MO diagram is the same as for the homonuclear molecules. It has a bond order of 2.5 and is a paramagnetic molecule.
When we make the molecular orbital energy level diagram of f2 molecule then, we will get this configuration: 1σs 2, 1σ*s 2, 2σs 2, 2σ* 2, σ2pz 2, π2p x 2, π2p y 2, πp x * 2, π2p y * 2. From this electronic configuration, we can see that there are a total of ten bonding molecular orbitals and eight antibonding molecular orbitals.
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
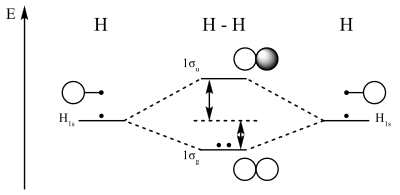
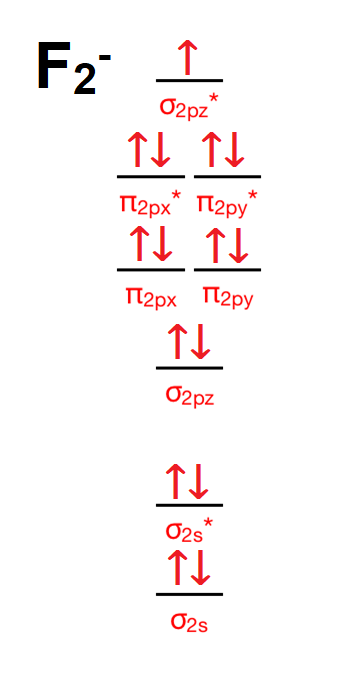
The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding.The ...
F2 MO Diagram. 207 views207 views ... Calculate Molecular Orbitals using WebMO. Eric Victor ... Molecular Orbital (MO) Diagram for F2(2+).
Total number of electrons in the F2^2- are calculated as. (7*2) +2 = 16 electrons. 2 is added because we have -2 charge on the molecule ion. Following image shows the number of electrons in the each molecular orbital. The calculated bond order is 0 for the F2^2-. Hottest videos.
39 molecular orbital diagram for h2o Written By Rosa B. Pruitt Tuesday, January 25, 2022 Add Comment Edit Mercury is the smallest planet in the Solar System and the closest to the Sun.Its orbit around the Sun takes 87.97 Earth days, the shortest of all the Sun's planets.
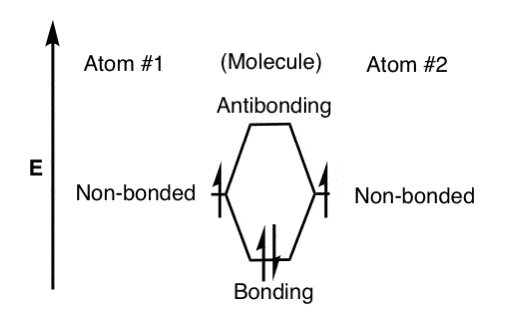
For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the ...
November 23, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...
Question: Draw a molecular orbital diagram for F2^2- . Calculate the bond order and magnetic behavior. This problem has been solved! See the answer ...
Determine the bond order from the molecular orbital diagram of N 2, F 2, and Ne 2. Does the bond order calculated agree with what you would draw for the Lewis structures of these molecules? Explain your answer here in addition to providing the bond order values. Learn this topic by watching MO Theory: Bond Order Concept Videos.
The molecular orbital electronic configuration, Magnetic property: Since bond order is zero, Be 2 molecule does not exist. It is diamagnetic due to the absence of any unpaired electron. B 2 molecule: The electronic configuration of B atom (Z = 5) is. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of ...
When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ...
Question: draw the molecular orbital diagram for F2 and F2+ - which one is paramagnetic/ diamagnetic - which one has a stronger bond. This problem has been solved! See the answer See the answer See the answer done loading. draw the molecular orbital diagram for F2 and F2+
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Group Valence Electrons F 7A 2 ... charge: +2 e– Total: 16 valence e– ... Use the molecular orbital diagram shown to determine which of the following is most stable. ... Q. What is the bond order of B2-?Is B2-paramagnetic or diamagnetic?a. paramagneticb. diamagneticc. Neither · Q. Determine the bond order for F2, F2+ and F2 ...
of bonding electrons - no. Is F2 2+ paramagnetic or diamagnetic? F₂²⁺ is paramagnetic. From the molecular orbital electronic configuration, number ...
Draw the molecular orbital diagram for F2 and find out the bond order 2 See answers Advertisement Advertisement DarkFrost DarkFrost O2 and F2 is an execption so their Z shell is down because of low energy level.but in other molecules like N2,H2 etc. The z shell is in higher level than pie2pxy because in other their energy level is more.
June 12, 2018 - You must generate a charge of +2. ... Below is a molecular orbital diagram for a fluorine molecule.
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
April 5, 2018 - Answer (1 of 5): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...
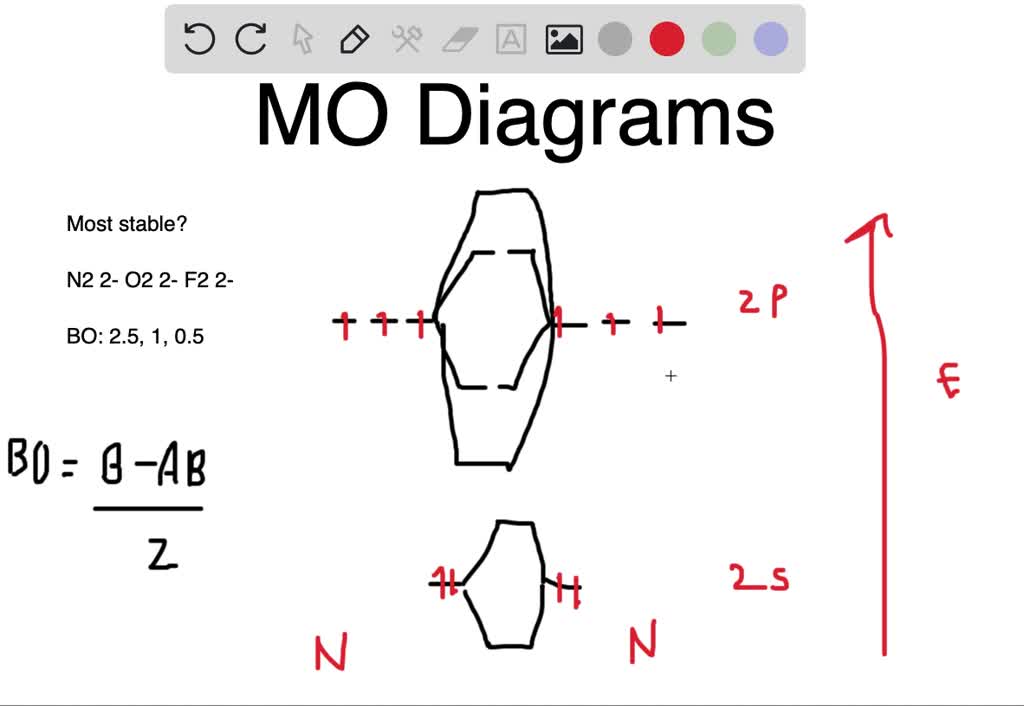
which of the following are predicted by the molecular orbital model to be stable diatomic species a h2 h2 h2 h2 2 b he2 2 he2 he2 c n2 2 o2 2 f2 2
June 12, 2020 - Click here👆to get an answer to your question ✍️ 37. Draw molecular orbital diagram for F2 molecule. Also, give its electronic configuration, bond order and magnetic property. 138. Solve the following:
For example, an ns/ns overlap for a homonuclear diatomic molecule gives rise to a partial MO diagram like this: and an np/np overlap for O2 and F2 gives: So, the full MO diagram is: Thus, the valence electron configuration is: (σ2s)2(σ* 2s)2(σ2pz)2(π2px)2(π2py)2(π* 2px)2(π* 2py)2. Answer link.
November 9, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...
A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the σ 1s bonding orbital; the electron configuration is [latex](\sigma_{1s})^2[/latex]. We represent this configuration by a molecular orbital energy diagram ( Figure 9 ) in which a single upward arrow indicates one electron in an orbital, and two (upward ...
Thank u..plz remember this short trick table for objectives..now kindly solve examples in your +2 book.6 answers · 61 votes: Here is the solution, %3E * For O2 molecule, %3E * For F2 molecule, Thanks for reading.
Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2 ...
The molecular orbital diagrams for molecules and ions are drawn from the order of increasing energies shown in the molecular orbital configuration. Always remember that the number of molecular orbitals formed must be equal to the number of atomic orbitals that were combined in the molecule.








![Molecular orbital diagram of [CoF 6 ] 3-complex with six p ...](https://www.researchgate.net/profile/Majid-Monajjemi/publication/257140982/figure/tbl1/AS:669050661257229@1536525516648/Molecular-orbital-diagram-of-CoF-6-3-complex-with-six-p-donor-ligands.png)





0 Response to "38 f2 2+ molecular orbital diagram"
Post a Comment