37 chemistry potential energy diagram
Reaction coordinate diagrams are derived from the corresponding potential energy surface (PES), which are used in computational chemistry to model chemical reactions by relating the energy of a molecule (s) to its structure (within the Born-Oppenheimer approximation ). This lesson explains how to interpret potential energy diagrams of single step reactions, and how to identify important features such as Heat of Reaction and...
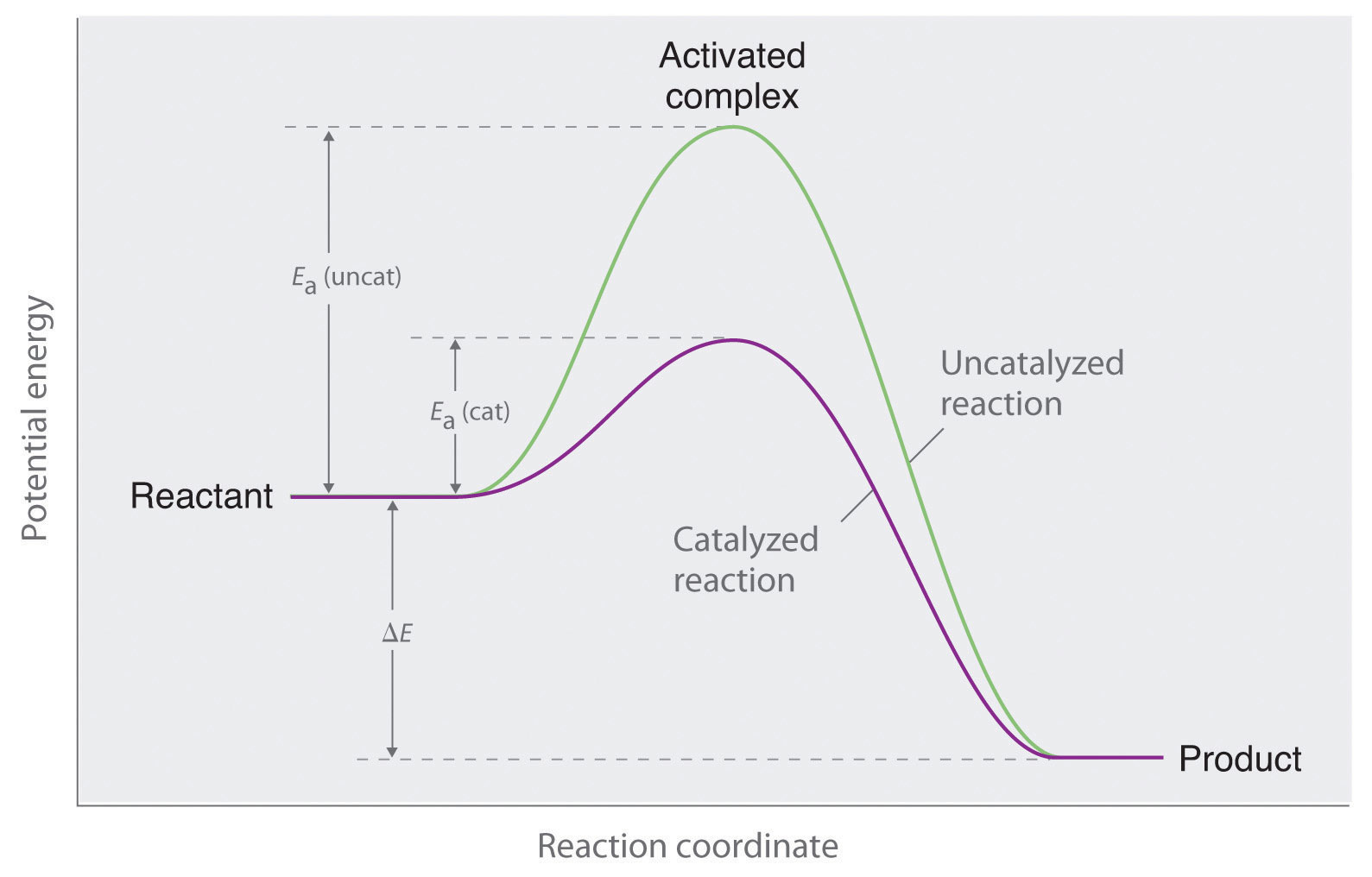
Chemical Potential Energy The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds. The reactants and products in a chemical reaction usually have different energy levels, which are shown in a energy level diagram.

Chemistry potential energy diagram
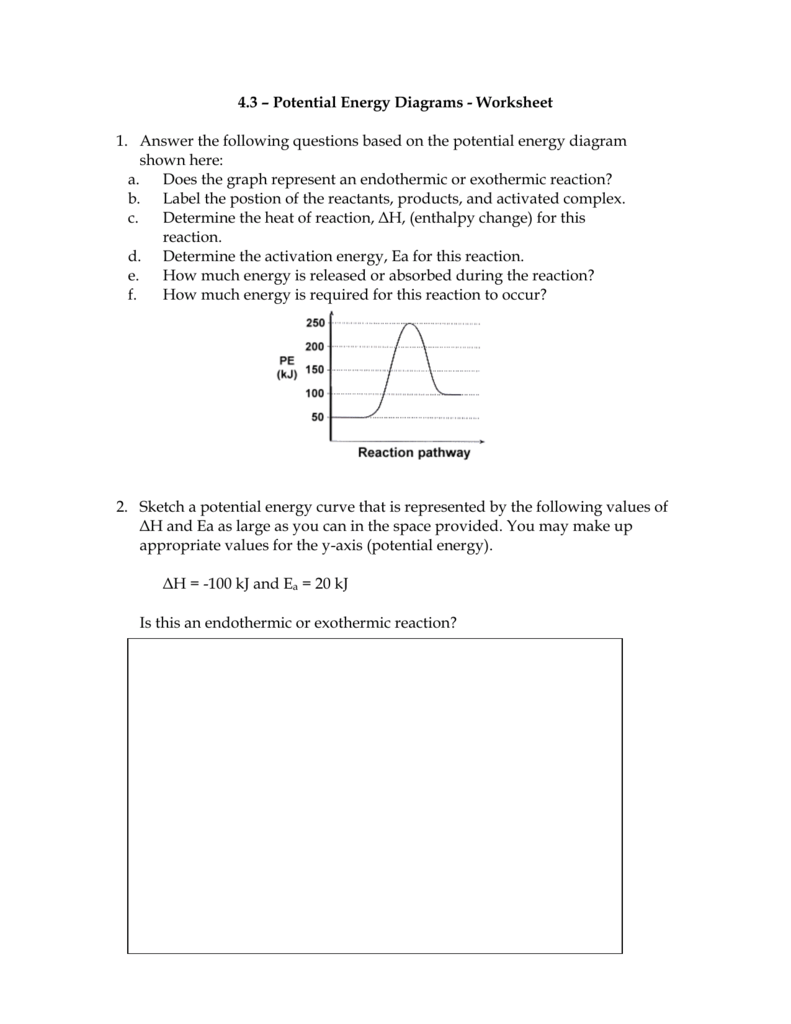
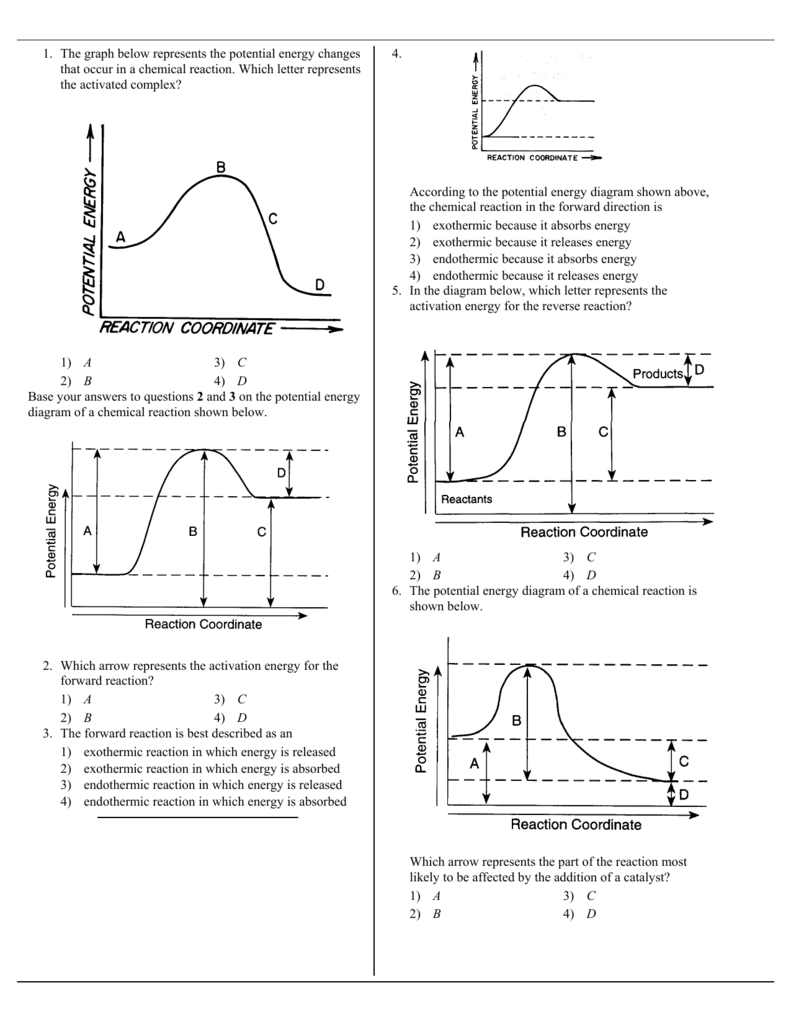
The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress curve. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. Chemistry 12 Potential Energy Diagrams Name: Date: Block: USE THE POTENTIAL ENERGY DIAGRAM TO ANSWER THE QUESTIONS BELOW: 1. Is the overall reaction as shown exothermic or endothermic? 2. What is the activation energy for the forward reaction? 3. What is the activation energy for the reverse reaction? 4. What is the enthalpy change of reaction (∆H) for the forward reaction? Potential energy diagrams Chemical reactions involve a change in energy, usually a loss or gain of heat energy. The heat stored by a substance is called its enthalpy (H). \ (\Delta H\) is the...
Chemistry potential energy diagram. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... Under this assumption, the activation energy barrier for A (higher energy conformation) should be less than that for B, which should explain the observed reaction rates. Having said that, I would not necessarily think that energy diagrams are the best way to explain this difference in reactivity, or indeed if there is a single, simple explanation. Potential Energy Diagrams A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Potential Energy Diagram Basics (PE Diagrams) Watch later Watch on How to draw the potential energy diagram for this reaction? Chemistry 2 Answers Truong-Son N. Apr 9, 2018 Since heat is released for C3H8(g) + 5O2(g) → 3CO2(g) +4H2O(g) + 2219.9 kJ, we say that ΔH ∘ C = − 2219.9 kJ/mol propane. We approximate that this is the change in potential energy for the reactants going to the products.
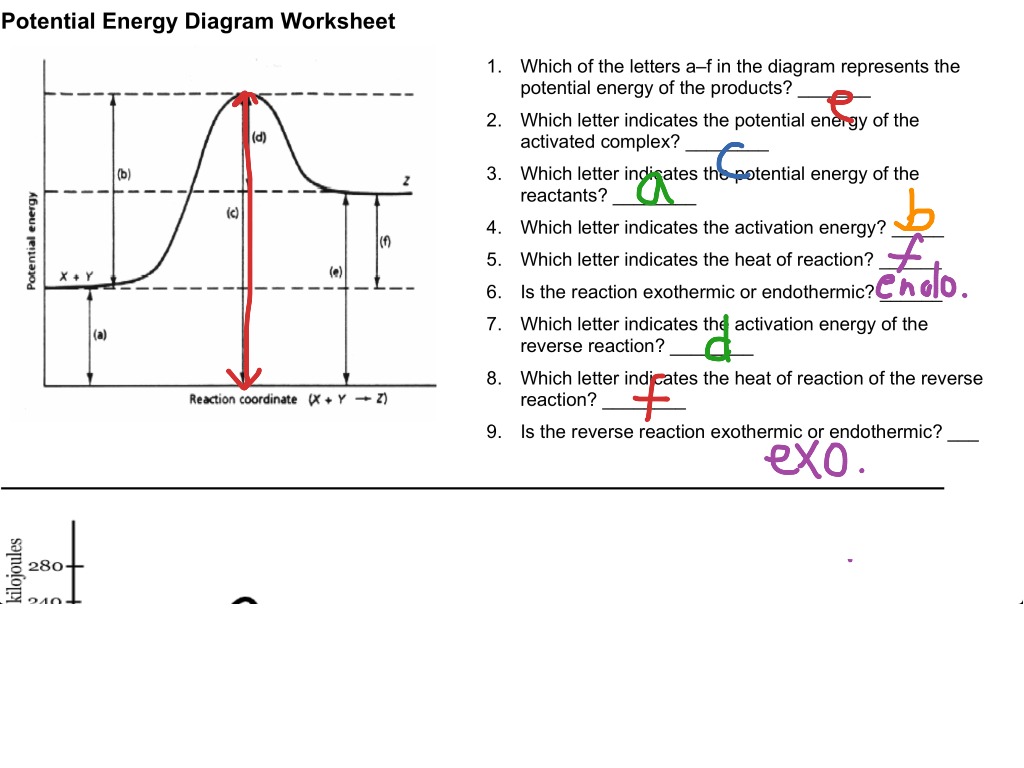
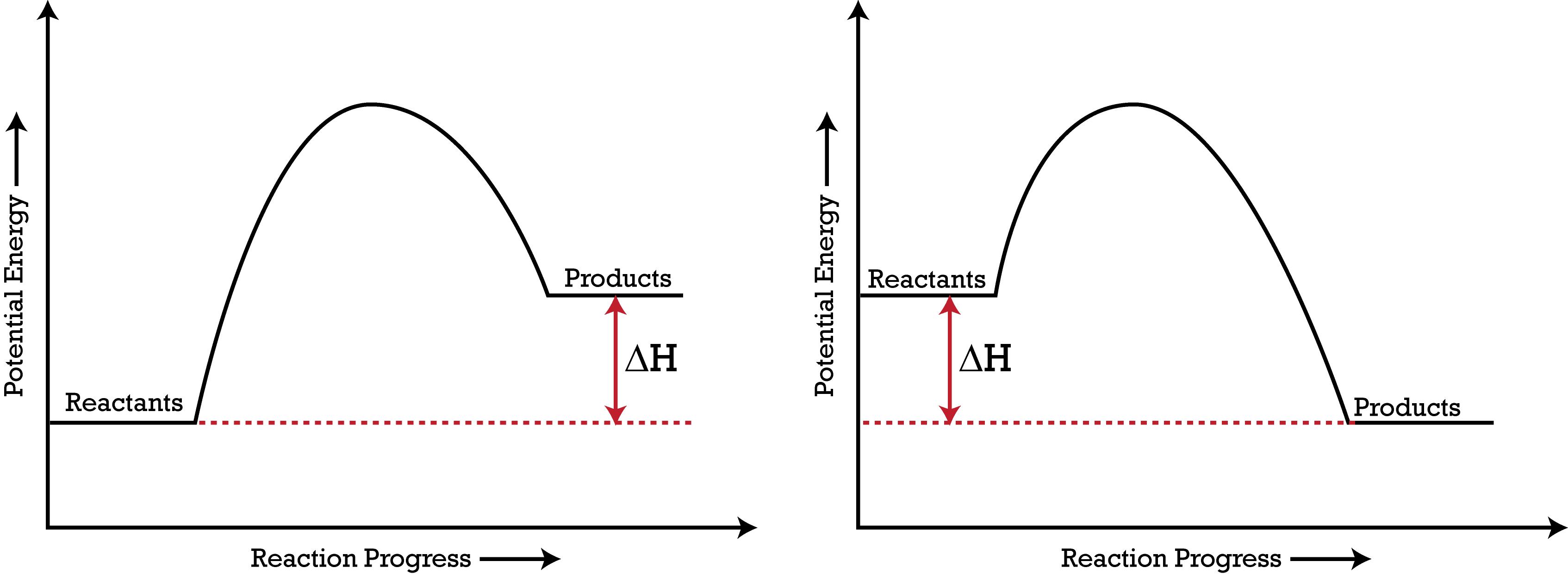
An Energy Profile is also referred to as an Energy Diagram or as a Potential Energy Diagram. An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what determines the rate at which the reaction occurs? Chemistry 12 Unit 1-Reaction Kinetics Worksheet 1-2 Potential Energy Diagrams Page 1 Chemistry 12 Worksheet 1-2 - Potential Energy Diagrams USE THE POTENTIAL ENERGY DIAGRAM TO ANSWER THE QUESTIONS BELOW: 1. Is the overall reaction as shown exothermic or endothermic? _____ 2. What is the activation energy for the forward ... More Kinetics Links Potential Energy Diagrams A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram.Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.
Customizable, digital textbooks. Adaptive Practice. Master concepts through Adaptive Practice. Study Guides. Use study guides to quickly review concepts. Schools. Find schools & districts near you on CK-12. Cafe. A place where you can ask, help, and share. potential energy, i.e., at a minimum on the PES. It sure would be nice to have ways of finding the locations of minima efficiently… • Between any two minima (valley bottoms) the lowest energy path will pass through a maximum at a saddle point. In chemistry, we call that saddle point a transition-state structure. They'd be nice to find too… Look back at the diagram. The 0 kJ/mol line represents the energy of the atoms when not bonded together, when they're bonded the potential energy of the atoms is lower than being unbonded. So there's no reason for the potential energy to "fall" again. ( 1 vote) Varun Patel a year ago Potential energy is energy stored in a system of forcefully interacting physical entities.. he SI unit for measuring work and energy is the joule (J).. The term potential energy was introduced by the 19th century Scottish engineer and physicist William Rankine.
Chemistry*12* Potential*Energy*Diagrams*Worksheet* Name:* Date:* Block:*! USE!THE!POTENTIAL*ENERGY*DIAGRAM!TO!ANSWER!THEQUESTIONS!BELOW:! 1.! Is!the!overall!reaction ...
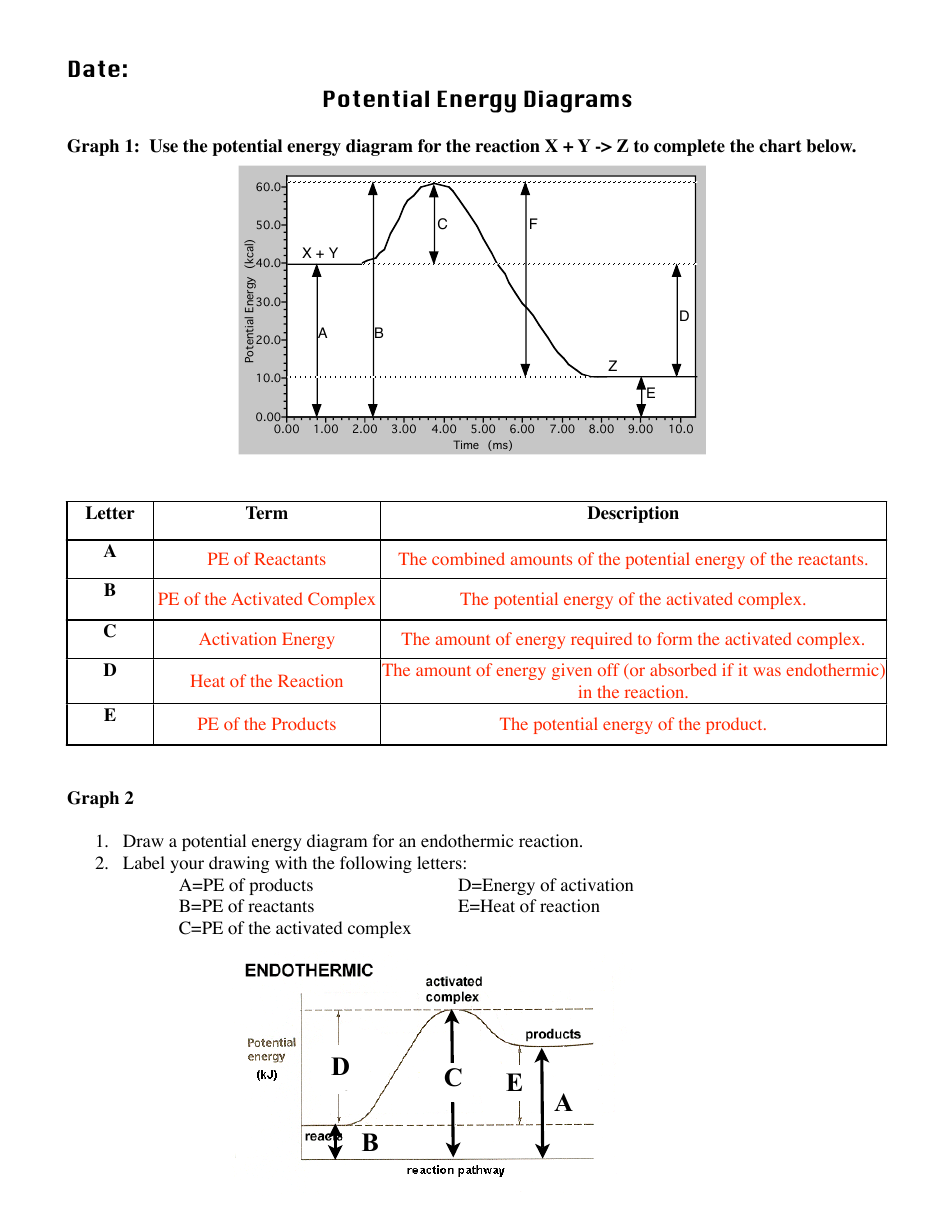
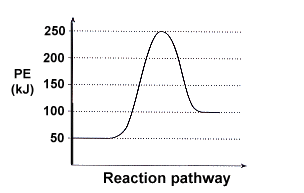
Potential energy diagrams are used to represent potential energy changes that take place as time progresses during chemical reactions. The potential energy diagrams for the exothermic reaction between substances X and Y is shown below. The reaction progresses from stage 1 (reactants),
POTENTIAL ENERGY DIAGRAMS ARE A VISUAL REPRESENTATION OF POTENTIAL. ENERGY IN A CHEMICAL REACTION. NOTE THE X AXIS IS USUALLY REACTION CORDINATE OR TIME.8 pages
How to Read Potential Energy Diagrams 1. THERMODYNAMICS: REACTION ENERGY 2. DAY 1 NOTES The Flow of Energy Energy - the capacity to do work or supply heat Chemical Potential Energy - energy stored within the bonds of chemical compounds Activation Energy - the minimum energy colliding particles must have in order to react
Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress curve. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products.
For covalent bonds, bond length is influenced by the bond order (single, double, triple) and the balance between repulsive and attractive forces. Bond length is the physical distance between two atoms bonded to one another. Bond energy in the diagram shows how the greatest potential energy is the repulsion of two atoms.
Chemistry 12 Unit 1-Reaction Kinetics Chemistry 12 Worksheet 1-2 - Potential Energy Diagrams USB THE POTENT!AT, ENERGY DIAGRAM TO ANSWTR THE OTJF.STTONS BFJ.OW: 5 >> E 5 a a. 130 100 0 X2Y2. 2XY PROGRESS OF REACTION 1. Is the overall reaction as shown exothermic or endothermic? 2. What is the activation energy for the forward reaction?

(Fang Ruida) Davis. K Coronavirus pneumonia biomarkers and treatment(方瑞达) Davis。K å† çŠ¶åž‹ç—…æ¯’æ€§è‚ºç‚Žç”Ÿç‰©æ ‡å¿—ç‰©åŠæ²»ç–—防治
Figure 1: A potential energy diagram for a toy car. The potential energy can arise from a variety of interactions including elastic, gravita-tional, chemical, etc. In this tutorial, the potential energy changes are generated with the help of magnets. However, the interactions between atoms and nuclei are of different origin.
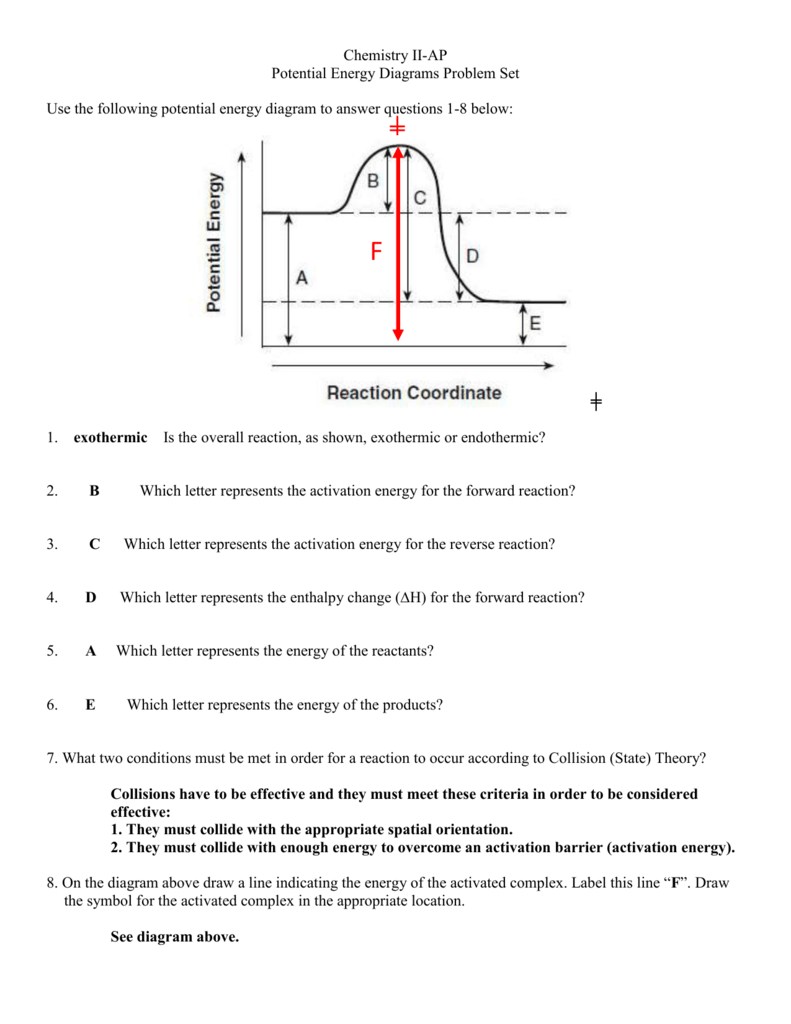
• Potential Energy diagrams represent changes in the potential energy of the reacting particles forming products when they are colliding. a) Activation Energy for the Forward Reaction o energy needed to form the activated complex. (or the energy needed to be overcome to form products).

Tree of life (Kabbalah)....Alkahest…Sleeping Prophet..chemical fortify the liver ( or failed) ...three main systems of roots, trunk and branches parallel human development of body, psyche and spirit.
1 | Potential Energy Diagrams Worksheet Potential Energy Diagrams Worksheet CK-12 Foundation Chemistry Name Use the following Potential Energy Diagram to answer questions 1 - 12. 150 100 Potential Energy X2+Y2 50 Progress of Reaction 1. Is the overall reaction as shown exothermic or endothermic? 2.
appear on the potential energy diagram? On this diagram the orbit of m 1 about m 2 is represented as a point with the coordinates 12 ( , ) ( , ) 00 2 mm r E r G r . From the graph we can tell that the orbit has a fixed radius 0r and a constant kinetic and potential energies. The potential energy is
Potential energy diagrams Chemical reactions involve a change in energy, usually a loss or gain of heat energy. The heat stored by a substance is called its enthalpy (H). \ (\Delta H\) is the...
Chemistry 12 Potential Energy Diagrams Name: Date: Block: USE THE POTENTIAL ENERGY DIAGRAM TO ANSWER THE QUESTIONS BELOW: 1. Is the overall reaction as shown exothermic or endothermic? 2. What is the activation energy for the forward reaction? 3. What is the activation energy for the reverse reaction? 4. What is the enthalpy change of reaction (∆H) for the forward reaction?
The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress curve. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products.



















0 Response to "37 chemistry potential energy diagram"
Post a Comment