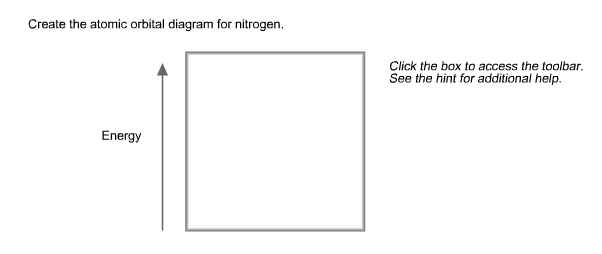
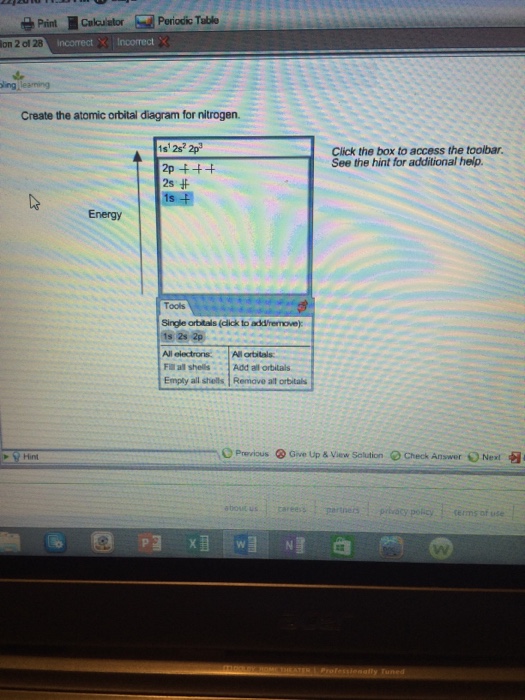
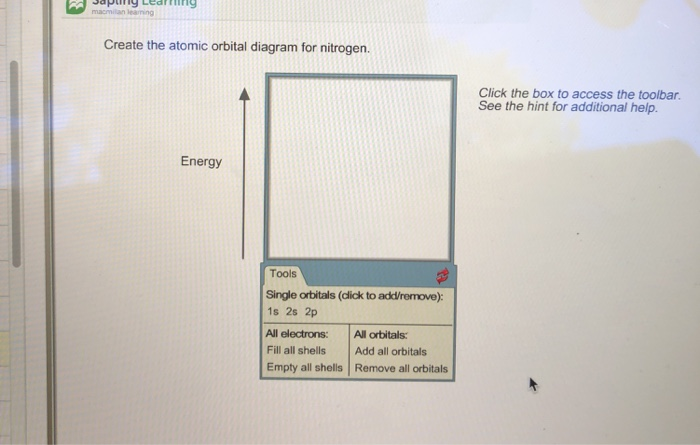
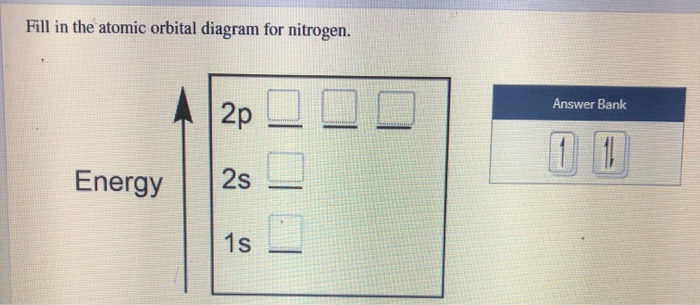
40 create the atomic orbital diagram for nitrogen
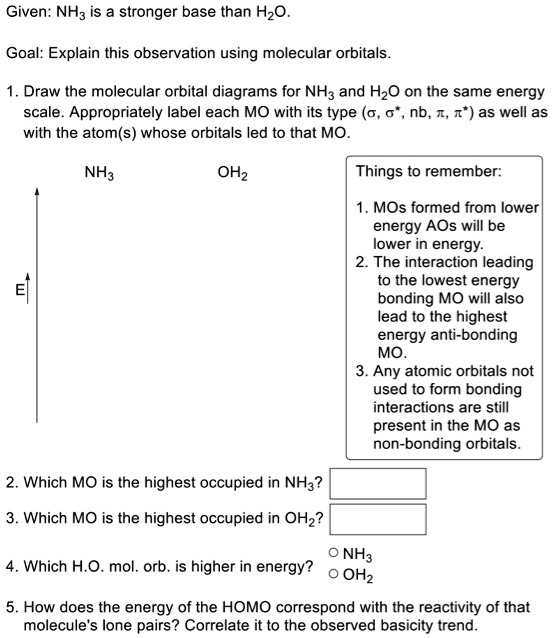
Here is the full molecular orbital diagram for N 2. Now we add the 10 electrons, 5 from each nitrogen atom. Note that the bottom sigma symmetry orbital is strongly bonding, the top one is strongly antibonding, and the 2 in the middle are only weakly bonding and antibonding, respectively.
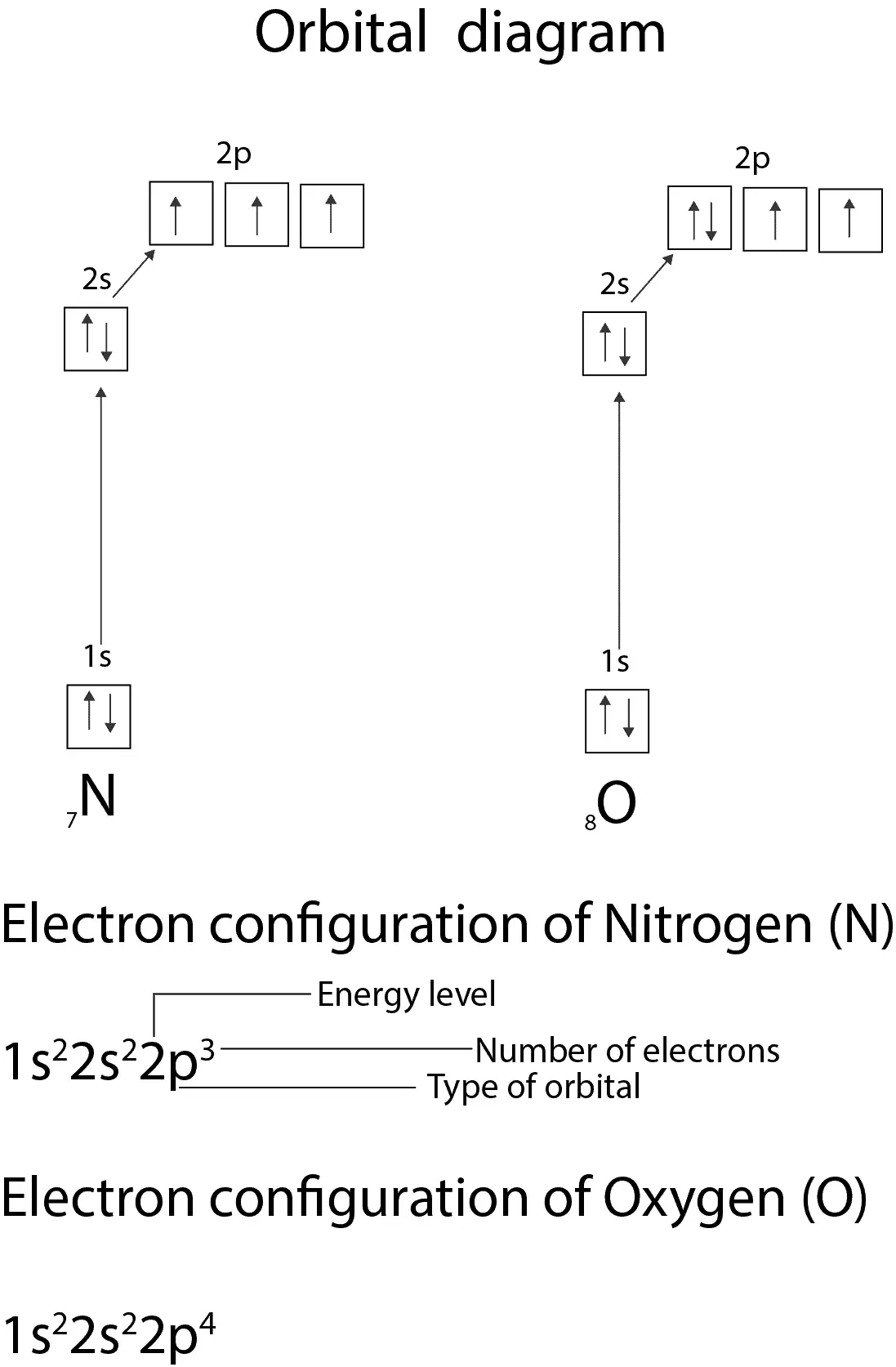
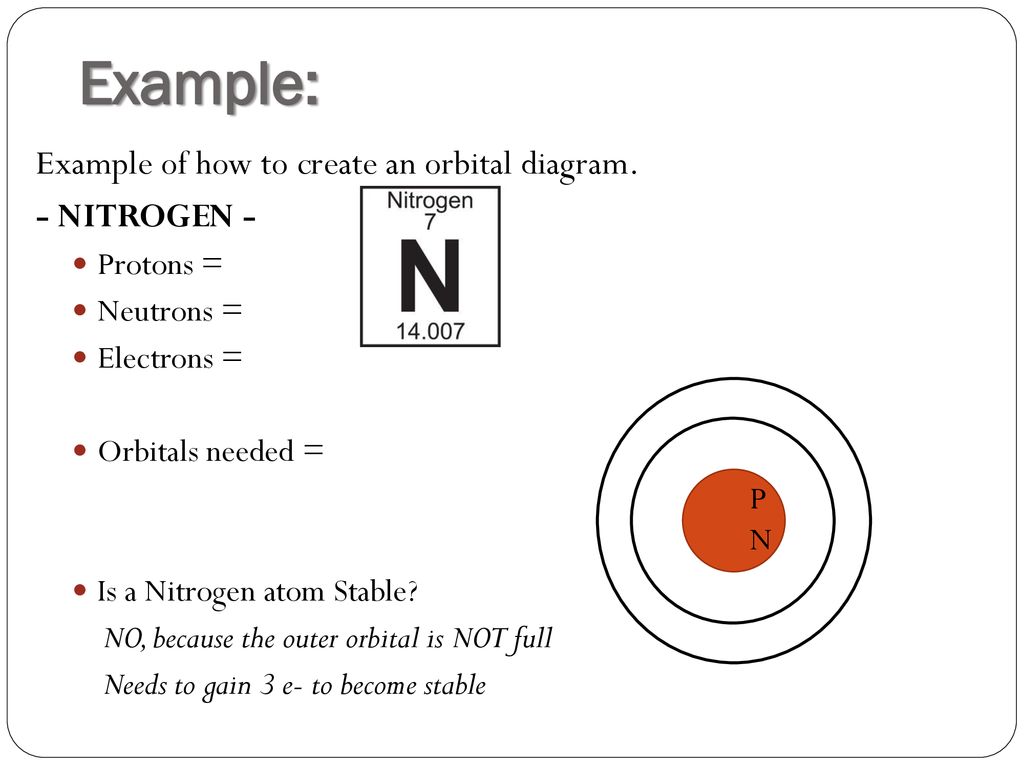
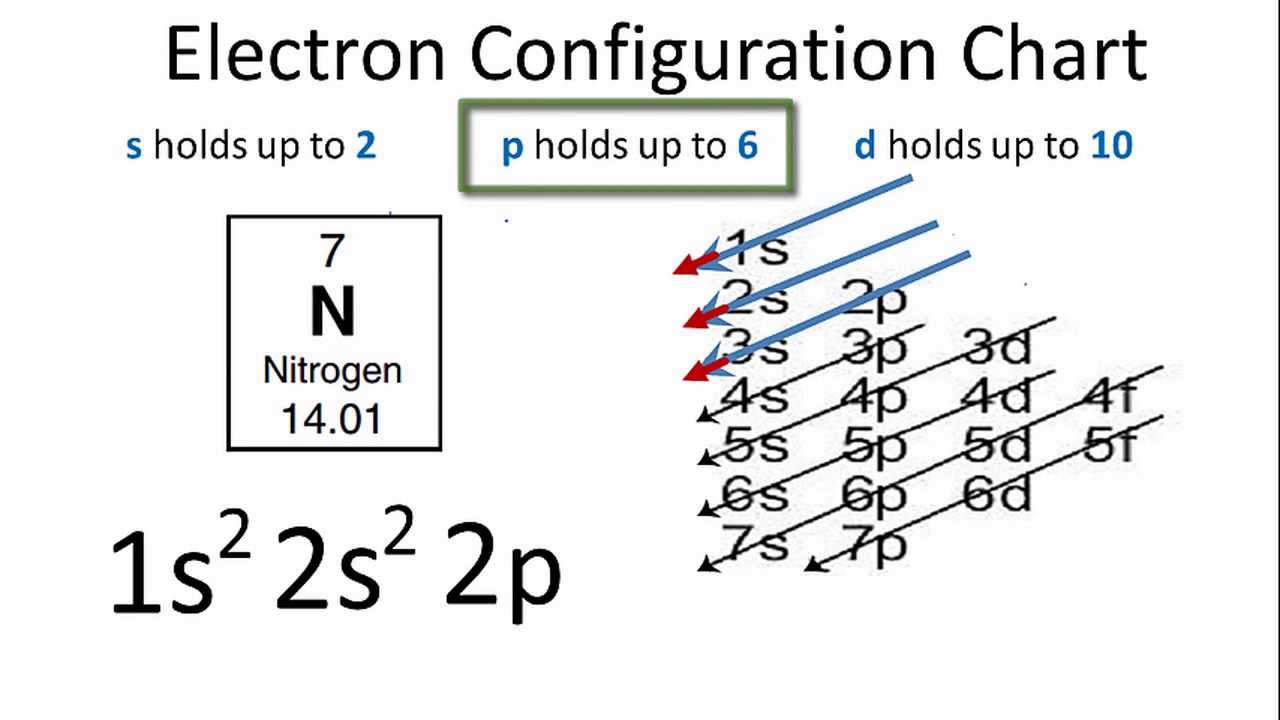
The orbital diagram for a ground state nitrogen is a a b b c c d d the electron configuration. It depends on the atom. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. The ground state electron configuration of p is ne3s23p3. Nitrogen is the seventh element with a total of 7 electrons.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...

Create the atomic orbital diagram for nitrogen
existence of this orbital, and often to know what it looks like. Atoms gain a lot by forming molecular orbitals. They have more stable arrangement for their electrons and the new bonds help them attain the nearest Noble gas configuration. In more advanced theory, every single atomic orbital can be considered, to some extent, in every
The molecular orbital energy level diagram of He 2 (hypothetical) is given in Fig. Here, N b = 2 and N a = 2. Bond order = N b -N a / 2 = 2-2 / 2 = 0. As the bond order for He 2 comes out to be zero, this molecule does not exist. 3. Nitrogen molecule (N 2). The electronic configuration of nitrogen (Z=7) in the ground state is 1s 2 2s 2 2p 1x 2p ...
Construct the orbital diagram of each atom or ion. The orbital diagram for a ground state nitrogen atom is. You may use the full electron shell notation or the inner shell noble gas method.
Create the atomic orbital diagram for nitrogen.
Construct the orbital diagram of each atom or ion. You may use the full electron shell notation or the inner shell noble gas method. Answer to the orbital diagram for a ground state nitrogen atom is. The orbital diagram for a ground state nitrogen atom. Write the electron configuration for the following elements.
Electron Configuration For Nitrogen Ion. The atomic number of nitrogen is 7, the element nitrogen was discovered by a Scottish physician, Danial Rutherford. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element Nitrogen.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
solved create the atomic orbital diagram for nitrogen st answer to create the atomic orbital diagram for nitrogen start by adding the appropriate subshells for example boron is in the create the atomic orbital diagram for nitrogen in some cases we may need to slightly alter the design color or even accessories we want a new thought for it then …
Orbital Theory Transformational properties of atomic orbitals Atomic orbital Transforms as s x2+y 2+z 2 px x py y pz z dz2 z2, 2z 2-x2-y2 dx2-y2 x2-y2 dxy xy dxz xz dyz yz S py • When bonds are formed, atomic orbitals combine according to their symmetry. • Symmetry properties and degeneracy of orbitals and bonds can be learned
Atomic Orbital Diagram: The simplified pictorial notation of the arrangement of the electrons within the different energy levels or orbitals of an atom is known as an atomic orbital diagram.
Create the atomic orbital diagram for nitrogen. Source: upload.wikimedia.org 2 aufbau principle electrons are added one at a time to the lowest energy orbitals available until all the electrons in an atom have been accounted for fully.
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
The three unhybridized 2p z atomic orbitals interact with one another to form three molecular orbitals: one \(\pi\) bonding orbital at lower energy, one \(\pi\)* antibonding orbital at higher energy, and a nonbonding orbital in between. Placing four electrons in this diagram fills the bonding and nonbonding orbitals.
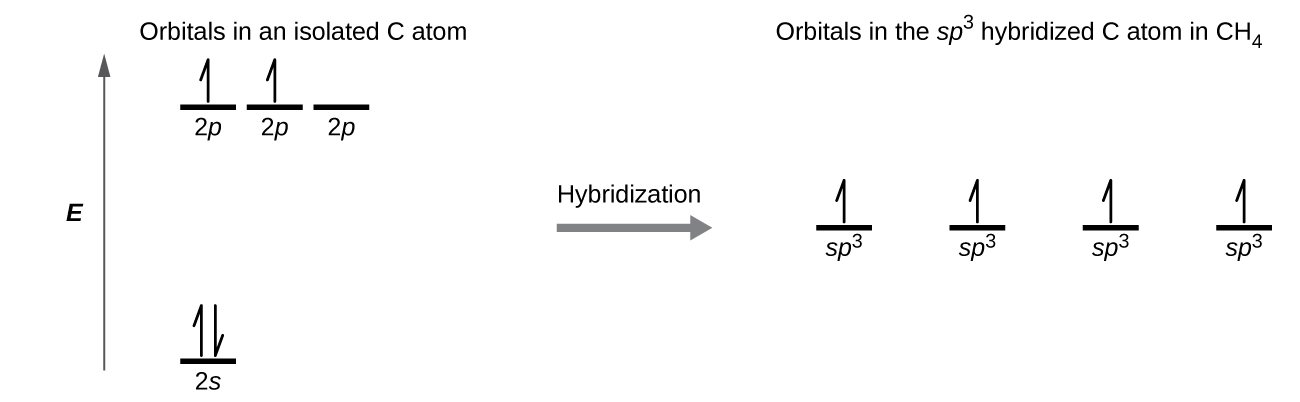
Figure 7.5.4. This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3p orbital. When atomic orbitals hybridize, the valence electrons occupy the newly created orbitals.
This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3p orbital. When atomic orbitals hybridize, the valence electrons occupy the newly created orbitals.
Create free Team Teams. Q&A for work. Connect and share knowledge within a single location that is structured and easy to search. ... (It is possible to construct more advanced orbital diagrams like this and this, too.) For the quantitative, ... Energies of atomic orbitals on molecular orbital diagrams. 37.
Answer to: create the atomic orbital diagram for nitrogen. By signing up, you'll get thousands of step-by-step solutions to your homework...
Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so you need to show the 2p subshell and everything below it. Next, click the orbitals to add electrons (represented as arrows). For boron, you would need to show a total of five electrons.
We will again need four hybrid orbitals, obtained by mixing one s and three p atomic orbitals in nitrogen. Nitrogen has five valence electrons ( ). Three hydrogen atoms with one unpaired electron apiece ( ) will overlap their 1 s orbitals with the three available sp3 orbitals on the nitrogen.
Create the atomic orbital diagram for nitrogen. Learn this topic by watching The Electron Configuration Concept Videos All Chemistry Practice Problems The Electron Configuration Practice Problems
This photo about: Atomic orbital Diagram Nitrogen, entitled as Create The Atomic Orbital Diagram For Nitrogen - Molecules Free Atomic Orbital Diagram Nitrogen - also describes Create The Atomic Orbital Diagram For Nitrogen - Molecules Free and labeled as: atomic orbital band structure,atomic orbital ground state,atomic orbital hybridization pdf,atomic orbital nitrogen,atomic orbital p ...
Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two ...
Create the atomic orbital diagram for nitrogen. Click the box to access the toolbar. See the hint for additional help. Question: Create the atomic orbital diagram for nitrogen. Click the box to access the toolbar. See the hint for additional help.
Q. Create the atomic orbital diagram for nitrogen. Q. Write the corresponding electron configuration for the following pictorial representation.Give the full electron configuration (do not use the noble...
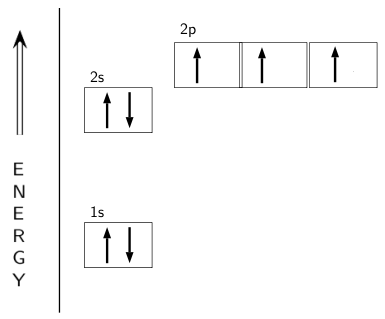
So the atomic orbital diagram is simply those orbitals in that order of energy. Note that the #1s# orbitals are significantly lower in energy than the #2s# orbitals. For the homonuclear diatomic #"O"_2# , we simply have two copies of this atomic orbital diagram far apart at first.
In a chlorine atom which subshells contain. Draw the atomic orbital diagram for chlorine. An orbital diagram is a sketch which shows electron population in atomic orbitals with the electrons. Create the atomic orbital diagram for chlorine. Create the atomic orbital diagram for nitrogen. The p orbital can hold up to six electrons.






















0 Response to "40 create the atomic orbital diagram for nitrogen"
Post a Comment