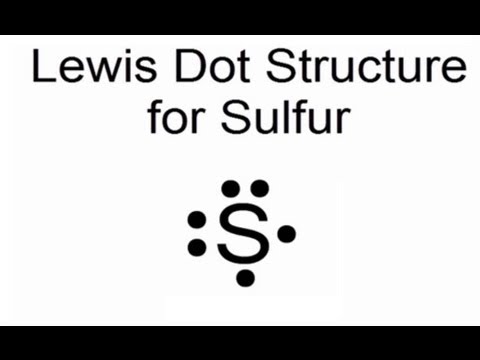
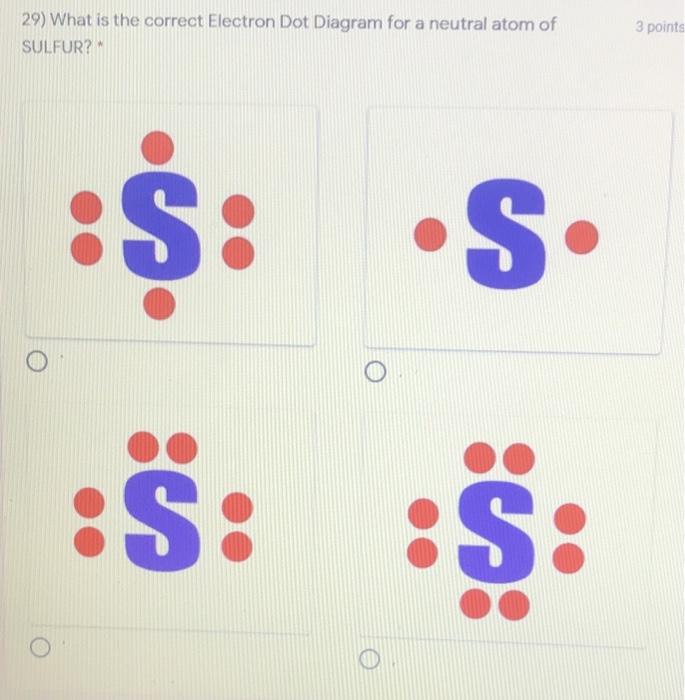
39 sulfur electron dot diagram
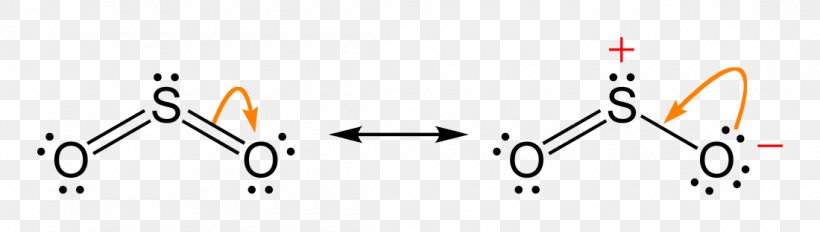
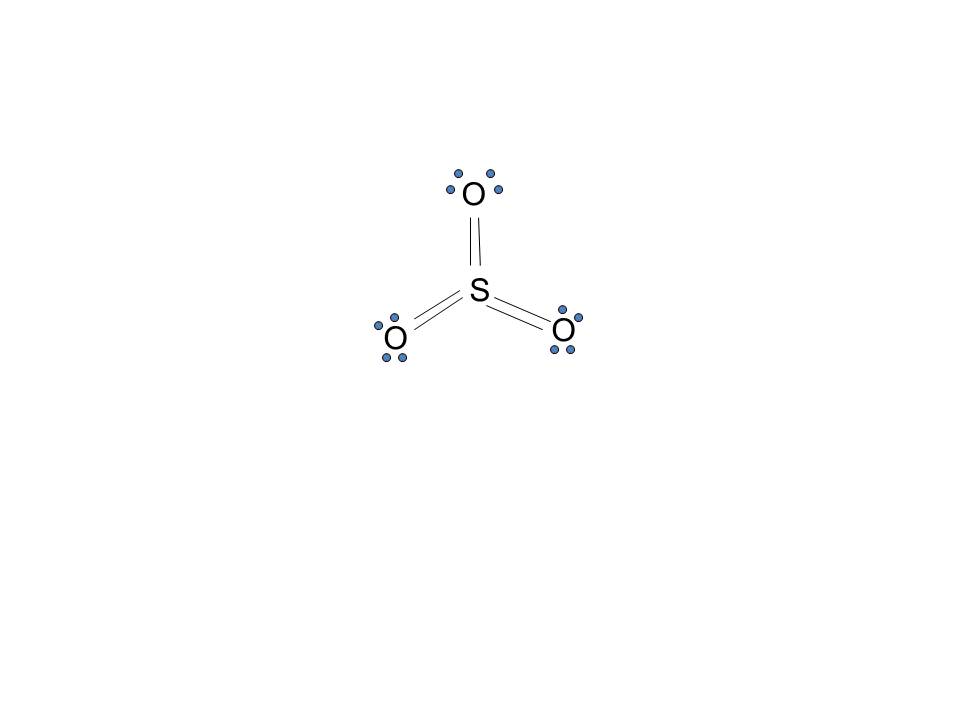
1. Draw an "electron dot" diagram showing the first 18 elements in the periodic table. 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of ions and ionic compounds. 4. Explain how hydrogen can be considered as behaving like a metal or a nonmetal. The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2.
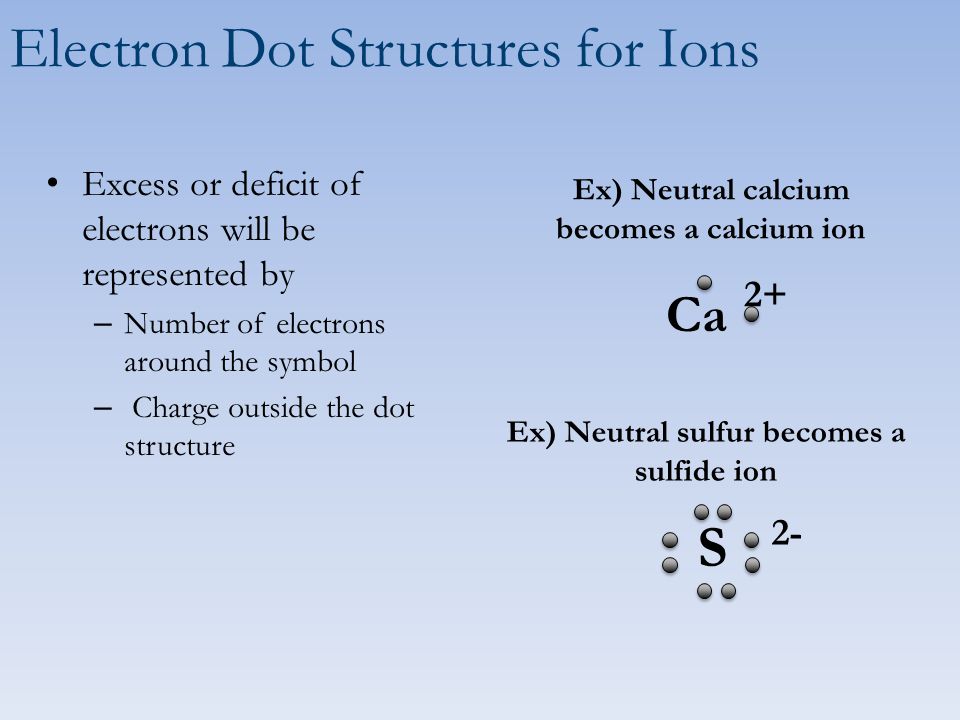
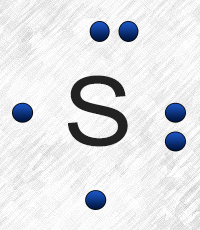
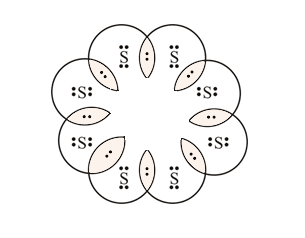
Sulfur belongs to group 16. It has 6 valence electrons. A \ 2- charge indicates that the atom has gained 2 electrons.
Sulfur electron dot diagram
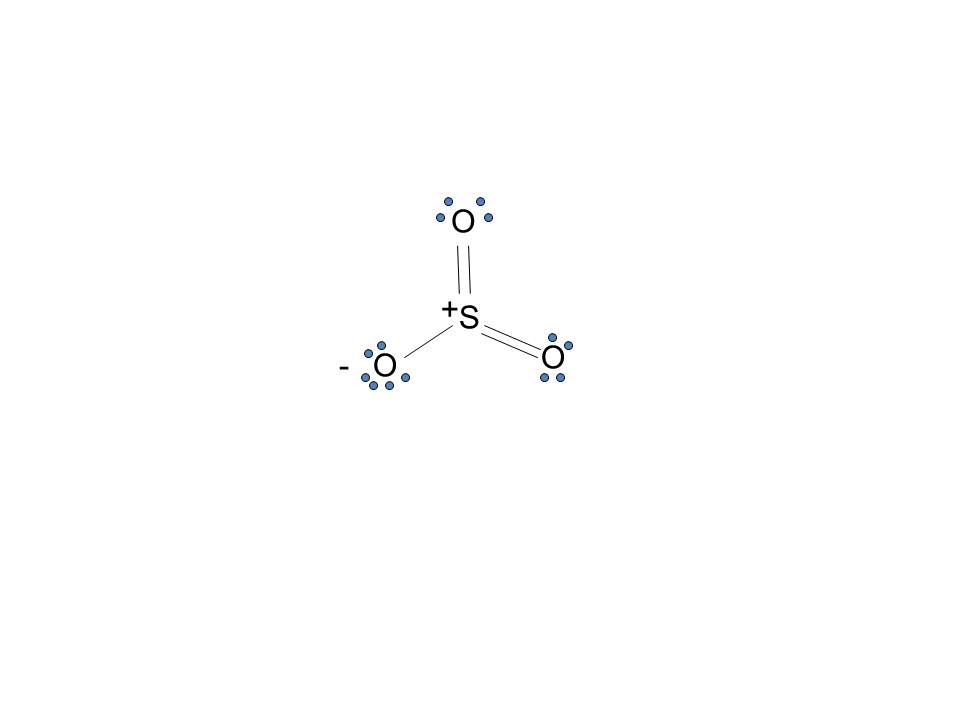
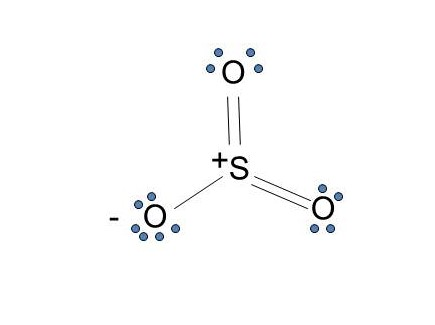
The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur. To draw the electron dot structure of atoms, we must know the atomic number of them. The total number of electrons represented in the Lewis dot structure will ... Answer: Draw the structure for the sulfite ion (SO3^2-) with the central sulfur having a single bond to each of two oxygens and a double bond to the third oxygen. Each of the single bonded oxygens also carries an extra electron (donated by the anion(s) in the sulfite compound). That gives the sul...
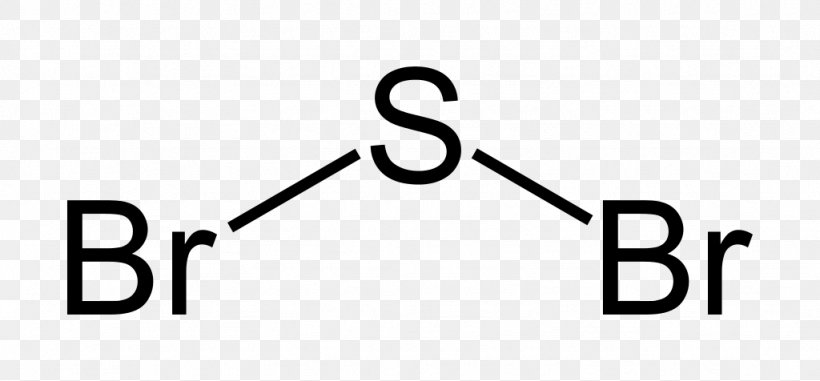
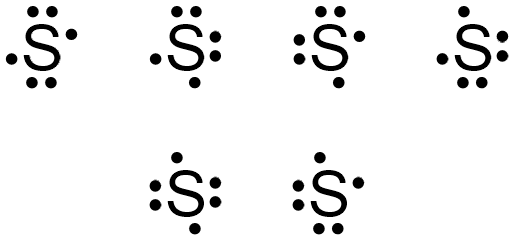
Sulfur electron dot diagram. For example, the Lewis electron dot diagram for calcium is simply ... of anions from atoms, as shown below for chlorine and sulfur: Two diagrams are shown. Purchase Handwritten notes of Class-X Science Ch-1 ,2 ,3 4 ,8 ,9,15 ... SBr2 Lewis structure is made up of two atoms, sulfur, and bromine, the sulfur is in the central position and bromine atoms are in the surrounding position. The lewis structure of SBr2 contains 16 nonbonding electrons and 4 bonding electrons. The lewis structure of SBr2 is similar to the SCl2 and it is very easy to draw. Here let's see how to ... The electron dot diagram for a lone uncharged Sulfur particle is an S with 6 electrons arranged around it (2 orbitals with 2 electrons and 2 orbitals with 1).
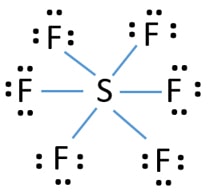
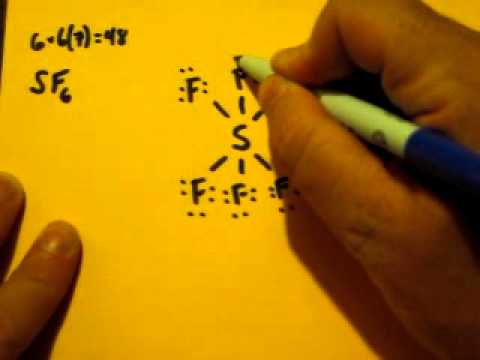
SF6 is a colorless and odorless gas that is non-combustible and non-flammable in nature. The central atom here is sulfur bonded with 6 fluorine atoms. Lewis dot structure has 6 sigma bonds and rests lone pairs on fluorine. The hybridization of SF6 is sp3d2. SF6 has octahedral molecular geometry and is non-polar in nature. Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms. Negative Electric Charge Electron Dot Diagram Polar Covalent Bond Covalent Bond Physical Science. TERMS IN THIS SET (14) electron dot diagram. a model of an atom in which each dot represents a valence electron. ion. an atom that has a net positive or negative electric charge. anion. an ion with a negative charge. The molecular geometry and polarity of Sulfur Monoxide Tetrafluoride, SOF4 using VSEPR rules. SOF 4 - Sulfur Monoxide Tetrafluoride: First draw the lewis structure: Electron geometry: trigonal bipyramidal. Hybridization: sp 3 d Second determine the 3D molecular geometry with VSEPR rules:
Answer (1 of 2): Lewis Structure for SO2 (Sulfur Dioxide)||Lewis Dot Structure of SO2 (Sulfur Dioxide) Hello,today I am going to draw the lewis structure for SO2 in just five steps. Step-1: To draw the lewis structure for SO2, we have to find out the valence electrons of sulfur and oxygen firs... When electrons are shared between each other, there is a formation of covalent bond. The atomic number of Sulphur is 16 and its electronic ... Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Sulfur, we got to know, it has 6 valence electrons. So, just represent the 6 valence electrons around the Sulfur atom as a dot. There are three electron groups around the central sulfur atom. These electron groups are (1) lone pair and (2) S-Br bonds. ... Just as the Lewis dot structure can visualize molecules, it can also ...
1: A valid electron dot structure for sulfur. The presence of unpaired electrons within an atom is inherently destabilizing. Therefore, if two ...
(b) On the basis of the Lewis electron-dot diagram that you drew in part (a), predict the molecular geometry of the IF 3 molecule. T-shaped One point is earned for the molecular geometry consistent with the Lewis diagram in part (a). (c) In the SO 2 molecule, both of the bonds between sulfur and oxygen have the same length. Explain this
Lewis dot structure of Nitride ion. Now let us try Lewis dot structure of Sulfide ion ( S 2-).Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). [Ne]4s 2 4p 6. Valence electrons are 8 (2 in 3s and 6 in 3p) Lewis dot structure of sulfide ion
Lewis Dot Diagram For So4 2. Simple procedure for drawing covalent Lewis structures - Lewis dot of the sulfate ion SO, best lewis structure for so, electron bonding. Viewing Notes: The Lewis structure for SO is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis.
Describe the electron dot diagram system of representing structure. Draw electron dot ... Why do oxygen and sulfur have the same electron dot structures?
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. It has a chemical formula of SF 2 and can be generated by the reaction of Sulphur Dioxide and Potassium Fluoride or Mercury Fluoride. In this blog post, we will look at the Lewis dot structure of SF 2, its molecular geometry and shape.
I. Draw Electron Dot Diagrams for the following elements. lithium oxygen neon magnesium iodine boron sulfur carbon phosphorus II. Draw Lewis Structures for the following molecules. ¥ PCl 3 CH 4 ¥ CH 3Br - e F 2O ...
A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how ...
SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity. Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among chemistry enthusiasts.
sulfur . LEWIS DOT DIAGRAMS Name Lewis diagrams are a way to Indicate the number of valence electrons aroun an atom. A\5Ö are all examples of this type of diagram. Draw Lewis dot diagrams of the following . l. calcium 2. potassiürn rgon 4. aluminum 2-3 -B 5, bromine 6. carbon . 7. helium 8. oxygen 9. phosphorus . hy rogen . Created Date:
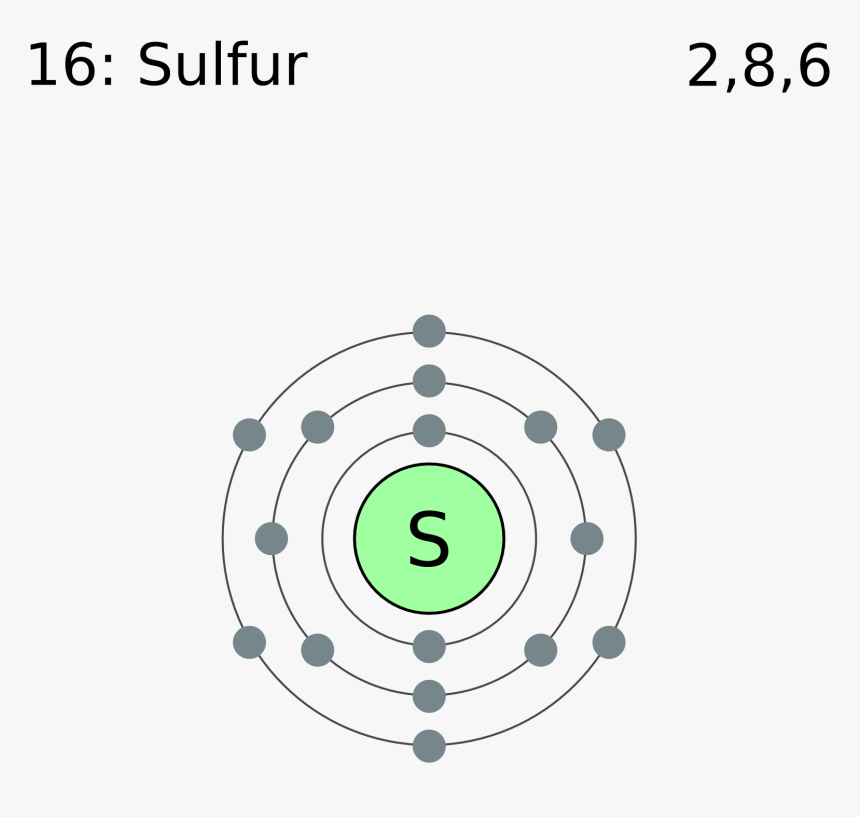
Therefore the sulfur electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 4. Video: Sulfur Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to ...
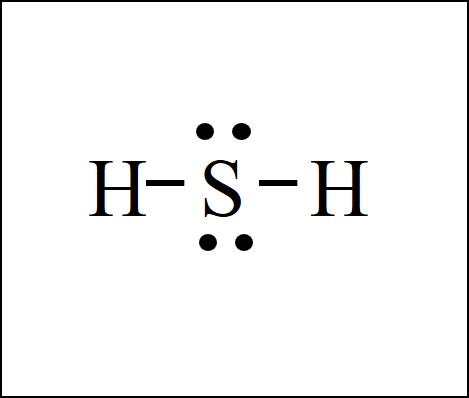
A Lewis Dot Structure is the diagrammatic representation of the bonding between the atoms of a molecule and the lone pair of electrons present in it. It is also known as electron dot structure/ Lewis dot diagram, Lewis dot formulas, Electron dot structure, or Lewis Electron dot structure (LEDs), respectively.
Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom.
Sulfur dioxide (SO 2) Lewis Structure, Hybridization. Sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. We will construct the lewis structure of SO 2 molecule by following VSEPR theory rules and considering stability of intermediate structures. After obtaining the lewis structure of SO 2, we can determine the hybridization of atoms.
SF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of 70.062 g/mol, this compound is made up of one Sulfur atom and two Fluoride atoms. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or ...
Refer to the explanation. Sulfur is in group 16/VIA, so its atoms have six valence electrons. The Lewis dot symbol for an element represents ...
A step-by-step explanation of how to draw the SI2 Lewis Dot Structure.For the SI2 structure use the periodic table to find the total number of valence electr...
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
Answer: Draw the structure for the sulfite ion (SO3^2-) with the central sulfur having a single bond to each of two oxygens and a double bond to the third oxygen. Each of the single bonded oxygens also carries an extra electron (donated by the anion(s) in the sulfite compound). That gives the sul...
To draw the electron dot structure of atoms, we must know the atomic number of them. The total number of electrons represented in the Lewis dot structure will ...
The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur.














0 Response to "39 sulfur electron dot diagram"
Post a Comment