39 molecular orbital diagram for n2 2-
Nov 21, 2018 · We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The ... Oxygen atom has 2s and 2p valence orbital s and 6 valence electrons: Each oxygen contributes 6, so we distribute 12 valence electrons into the molecule to get "O"_2. Two 2s orbital s combine to give a sigma_ (2s) bonding and sigma_ (2s)^"*" antibonding MO. Molecular orbital diagram for o2- ion. When two oxygen atoms overlap, the sigma (2p ...
Molecular Orbital Diagram of O 2 Chapter 9 Section 6 When filling the MO levels, you have to: Count the number of valence electrons, Start with the lower energy orbital s first, Follow Hund's rule, and Pt t th t Dr. A. Al-Saadi 19 Put not more than two electrons in one MO.

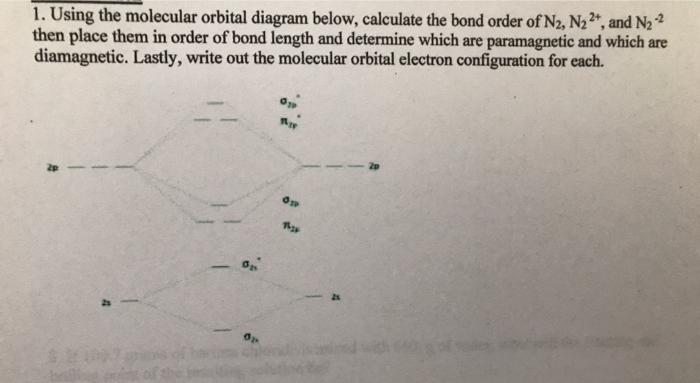
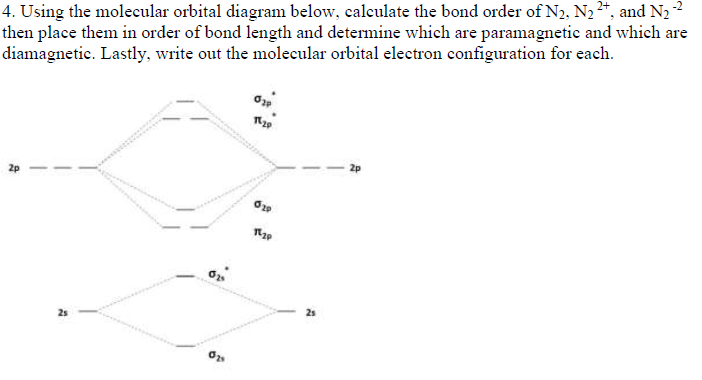
Molecular orbital diagram for n2 2-
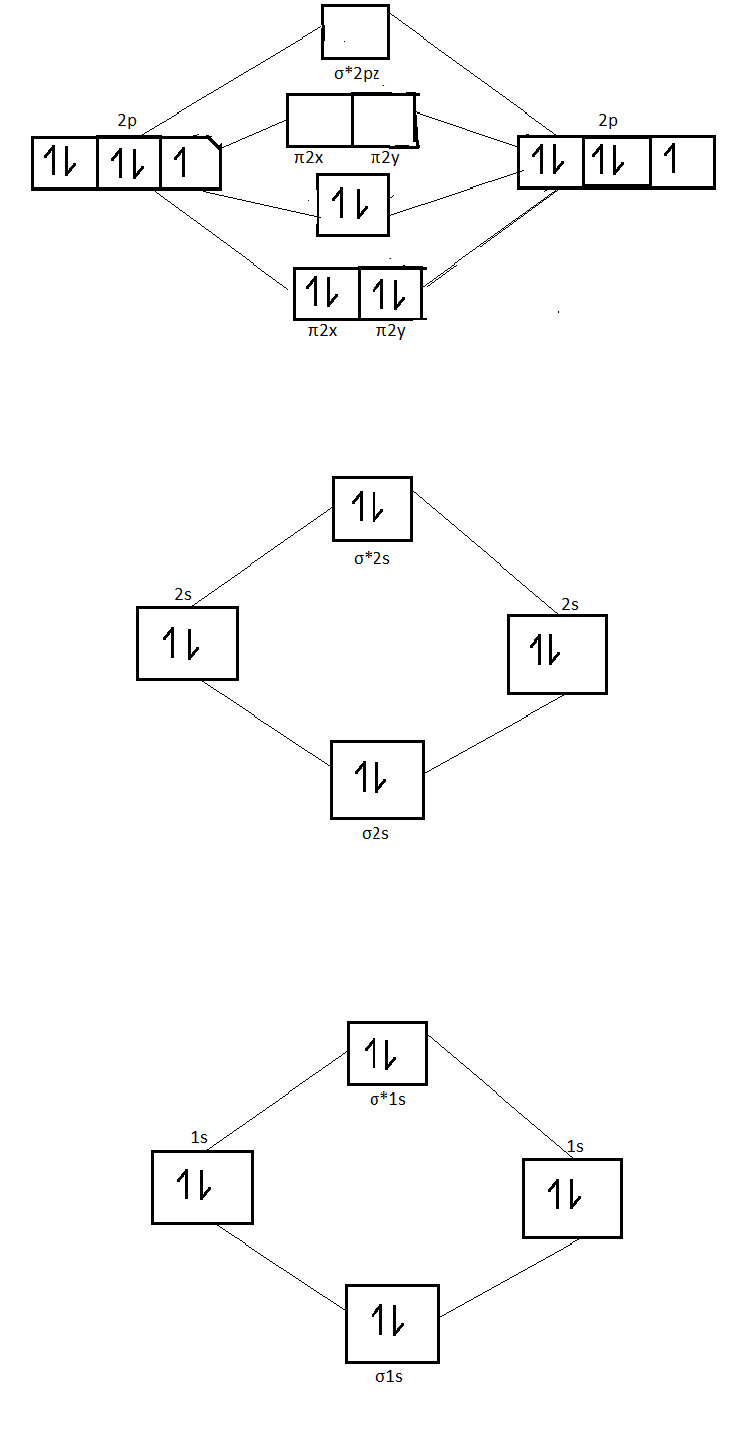
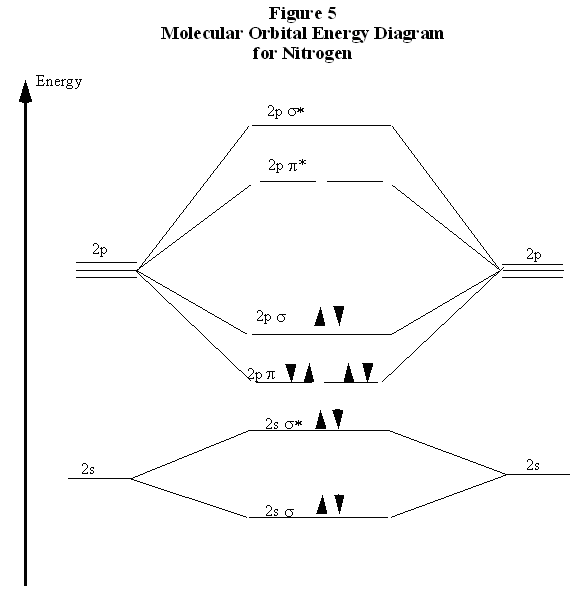
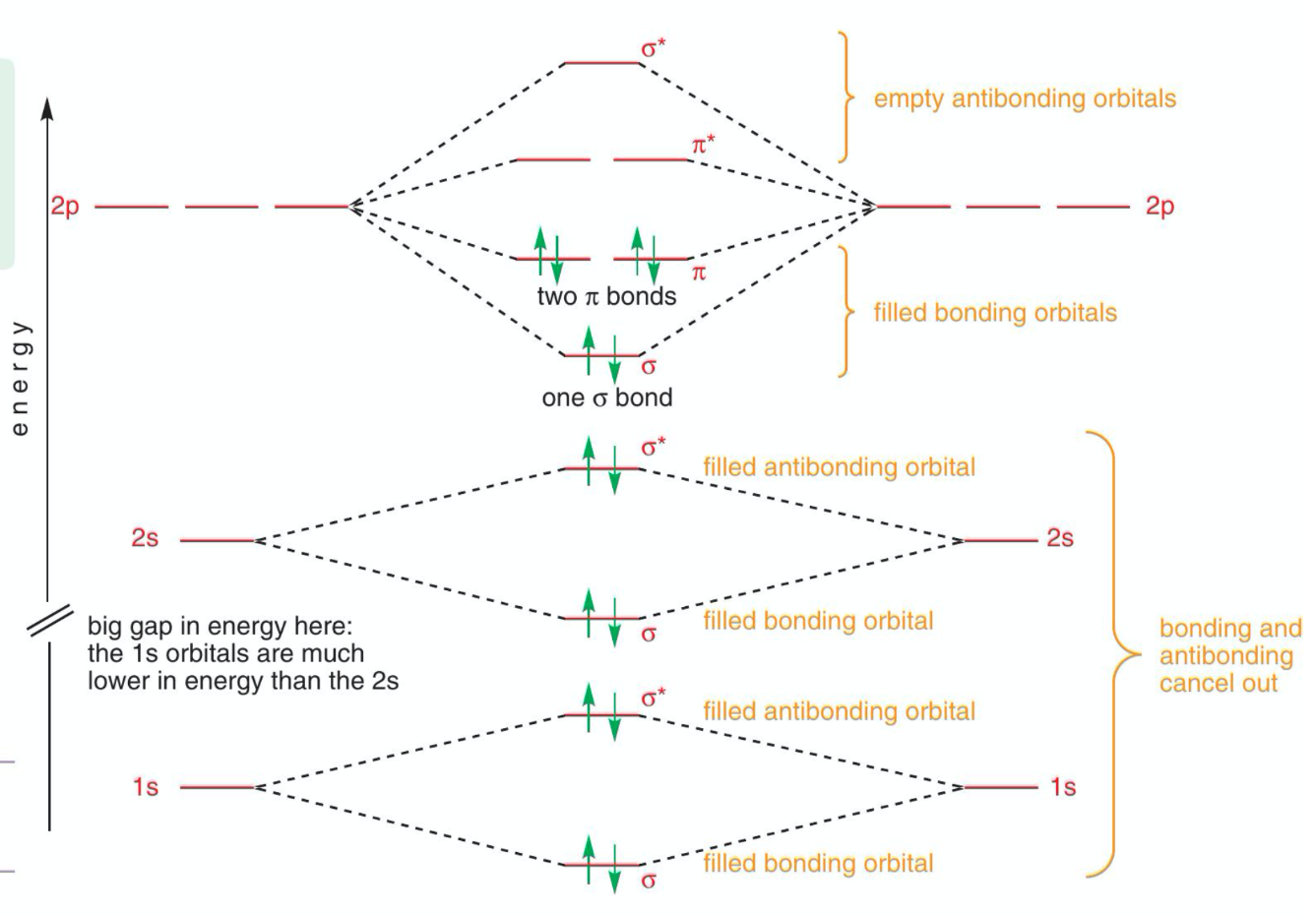
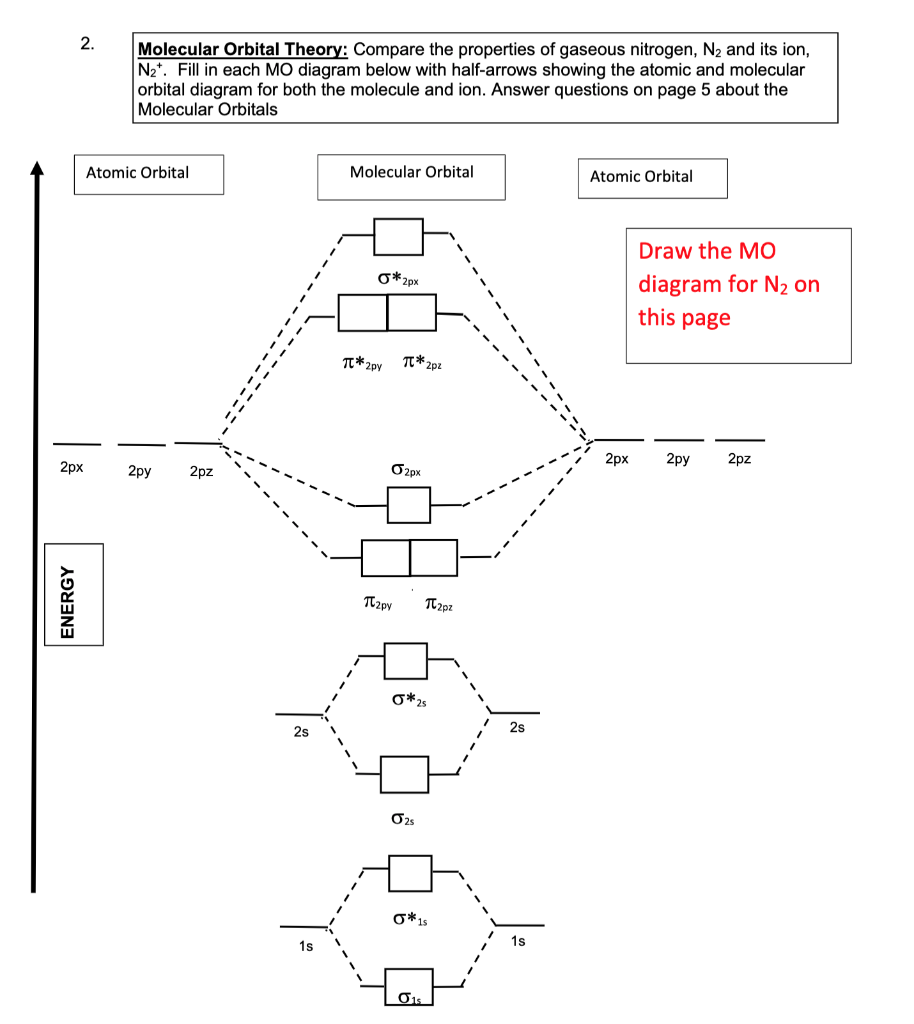
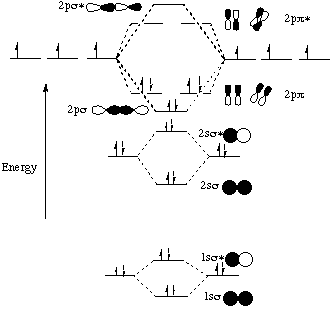
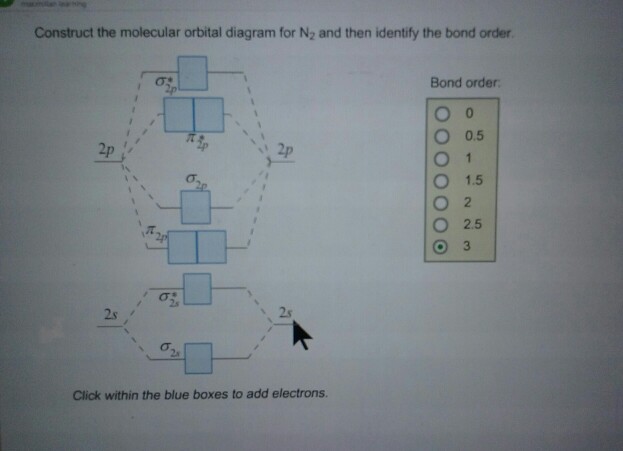
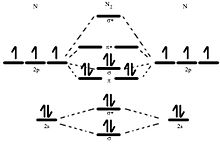
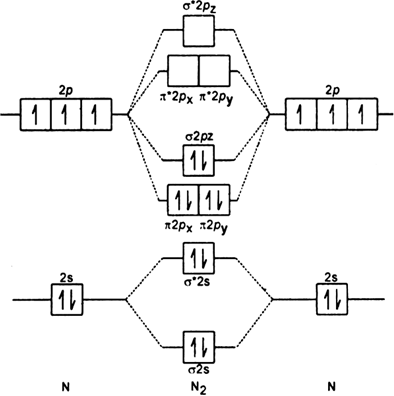
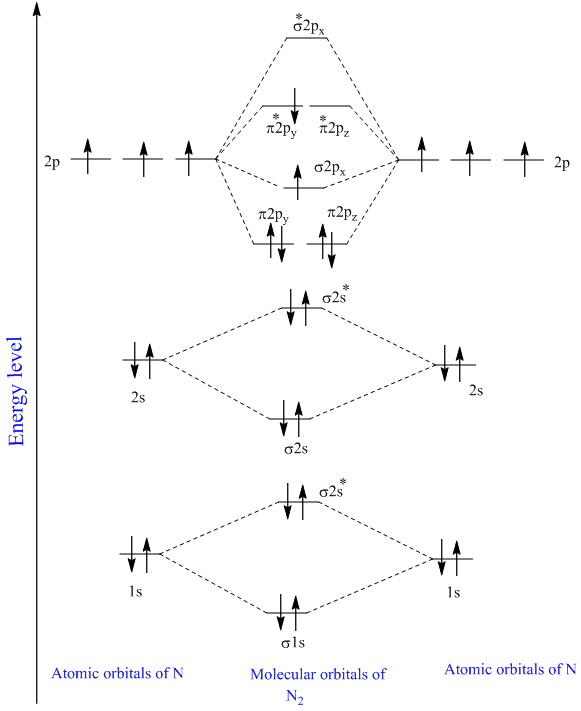
Molecular orbital diagram of dinitrogen molecule, N 2. There are five bonding orbitals and two antibonding orbitals (marked with an asterisk; orbitals involving the inner 1s electrons not shown), giving a total bond order of three. Atomic nitrogen, also known as active nitrogen, is highly reactive, being a triradical with three unpaired electrons. N2 Molecular Orbital Diagram. With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more familiar diagram. Notice how the σ from the 2p behaves more non-bonding like due to mixing, same with the 2s σ. This also causes a large jump in energy in the 2p σ* orbital. Ionic character in covalent compounds: Bond moment and dipole moment. Percentage ionic character from dipole moment and electronegativity difference. Molecular orbital theory. Molecular orbital diagrams of diatomic and simple polyatomic molecules N2, O2, C2, B2, F2, CO, NO, and their ions; HCl (idea of s-p mixing and orbital interaction to be ...
Molecular orbital diagram for n2 2-. Molecular orbital diagram for b2. By drawing molecular orbital diagrams for b2 c2 n2 o2 and f2 predict which of these homonuclear diatomic molecules are magnetic. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. Jun 05, 2017 · General Notes on Molecular Orbital Diagrams. The Y-axis of a MO diagram represents the total energy (not potential nor Gibbs Energy) of the orbitals. Individual atomic orbitals (AO) are arranged on the far left and far right of the diagram. Overlapping atomic orbitals produce molecular orbitals located in the middle of the diagram. 37 diagram sentences for me; 42 molecular orbital diagram for n2 2-38 nerd geek venn diagram; 38 amana dryer belt diagram; 39 cantilever umbrella parts diagram; 38 97 buick lesabre belt diagram; 42 2012 ford f250 tail light wiring diagram; 37 2012 ford focus parts diagram; 40 blank three circle venn diagram; 40 t5 transmission parts diagram N2H2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. Dinitrogen dihydride has the chemical formula of N2H2. This compound is most commonly known as diazene or diimide. It is a yellowish-colored gas having both cis and trans isomers. It can be prepared from the decarboxylation of azodicarboxylic acid ( (NCOOH)2 ).
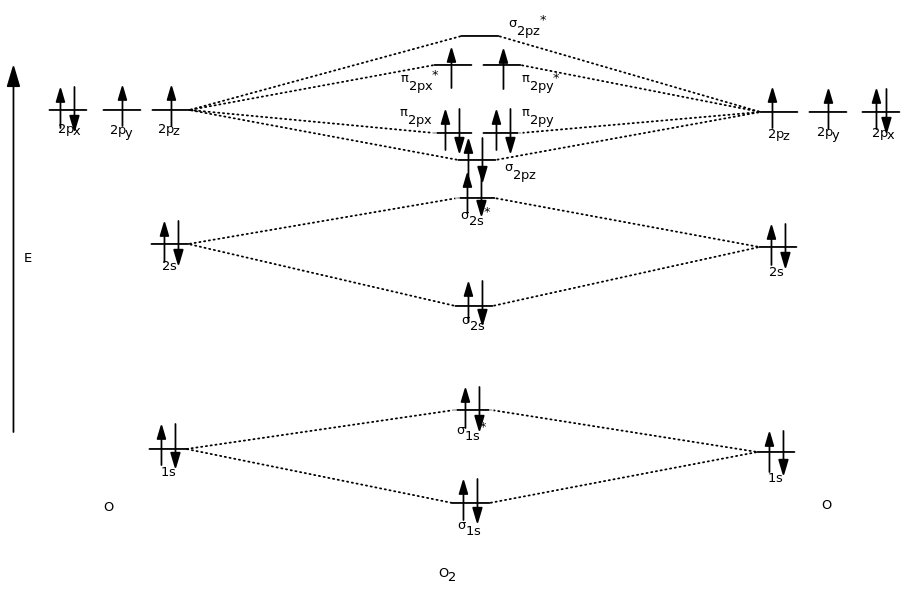
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic B2 Place the following in order of decreasing X-A-X bond angle, where A represents the central atom and X represents the outer atoms in each molecule. Molecular Orbital Diagram s. This scheme of bonding and antibonding orbital s is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbital s before the higher ones, just as is the case for atomic ... Molecular Orbital Diagram Of C2, N2 , O2 ,F2. Bromine - Br 2. At room temperature, Bromine is a red-brown liquid. This element forms diatomic molecules when the temperature is increased and it becomes gaseous. The outermost layer consists of 7 electrons, and needs one to become stable and make Br 2. Iodine - I 2. Iodine exists as a purple-black non-metallic solid at room temperature. The bond length in the oxygen species can be explained by the positions of the electrons in molecular orbital theory. To obtain the molecular orbital energy-level diagram for O 2, we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure 9.10.1 . We again fill the orbitals according to Hund's rules ...
Molecular orbital s: Orbital s that span two or more atoms. These are constructed by overlapping atomic orbital s (AOs) which match in symmetry and size. In principle, To construct MO diagram of a any Molecule, first, set up Schrödinger wave equation for that molecule and then, solve it!!! Molecular Orbital Diagram Maker. These quizzes enable you to build your own molecular orbital diagram ... Nov 17, 2021 · Molecular Orbital Diagram of N2 Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule. Answer (1 of 6): O2 2- bond order = 1 O2 - bond order = 1.5 O2 bond order = 2 O2+ bond order = 2.5 O2 2+ bond order =3 I hope it’ll work out :) Molecular orbital diagram of b2.Give bond order and predict whether they are diamagnetic or paramagnetic for all the molecules above question 1 3. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of ... Molecular Orbital s of the Second Energy Level.
Farmall 140 parts diagram. It shows the parts of the circuit as streamlined forms and the power as well as signal connections between the gadgets. It's either fuel or electrical. 2 in stock! FORD NEW HOLLAND 8160 8360 TM 120 130 140 IHC MXM 130 140 SERIES DUEL COMMAND SYNCHRONIZER (BIG CENTRE) Price: €564. Amazon.com: Farmall 140
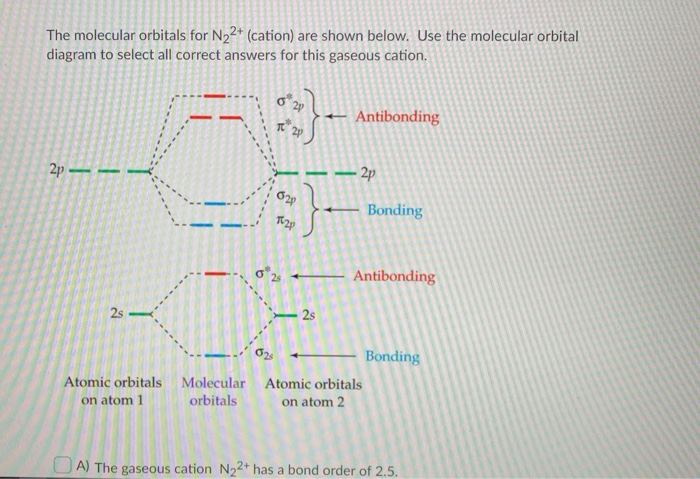
Answer (1 of 4): Hi guys let me tell u one short trick for finding bond order… First of all for the given molecule add up the total no of electrons present in that molecule.. For example here in N2 we have 7+7 =14 electrons. Now remember this table.. ELECTRONS = BOND ORDER 10 = 1 11 = 1.5 ...
A 5.31 g sample of compound that contains only hydrogen and sulfur was combusted, and yielded 10.0 g of SO₂ and 2.81 g of H₂O. The molar mass of the compound is 68 g/mol. What is this compound's molecular formula? A) H₄S₂ B) H₂S C) HS D) H₂S₂
Molecularorbitaldiagramfor n2 o2 c2f2 also h2o. Molecularorbitaldiagramfor carbon dimer c2. Mo diagrams can be used to deduce magnetic properties of a molecule and how they change with ionization. Molecularorbitaldiagramfor n2 o2 c2f2 also h2o. Electronic configuration of c2molecule is σ 1s2 σ1s2 σ2s2 σ2pz 2 2px 1 2py orbitals ...
A molecular orbital explicitly describes the spatial distribution of a single electron orbital s, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining ...
Molecular orbital diagram for o2- ion 1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881). 1834, introduced by English physicist and chemist Michael Faraday (suggested by the Rev. William Whewell, English polymath), coined from Greek ion ...
Calculate a molecules bond order given its molecular orbital diagram. No special bond order formula is usually required. Bond order is defined as half the difference between the number of bonding and antibonding electrons. For instance in diatomic nitrogen NN the bond order is 3 while in acetylene HCCH the bond order between the 2 carbon atoms.
Answer (1 of 3): I modified the picture from this post: What's the MOT diagram of O2 +2 ion? and modified it to be O2 2+ (since sadly enough I am about as advanced with artistic programs on pc as a rock). How you basically do these questions is by first drawing the empty AO and MO, then counting ...
You can also simply prove it by molecular orbital theory. What is the bond order for N 2? The MO method for N2+ gives the bond order equal to 2.5. But first, we look at the diagram of molecular orbitals for N2 (the bond order for the nitrogen molecule is 3).
09/06/2017 · molecular orbital diagram for nitrogen gas (n2)use aufbau and hund to fill with 10 valence electronsyou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).bond or. For the second period elements the 2s and 2p orbitals are important for mo considerations. 31/03/2021 · 12+ n2 molecular orbital diagram. 07/08/2021 · do you know !!
Molecular orbital diagram for n2 o2 c2 f2 also h2o. Fill from the bottom up with 8 electrons total. Mo diagram s can be used to deduce magnetic properties of a molecule and how they change with ionization. O2 2 Molecular Orbital Diagram Fabulous Electron Molecular Orbital. Molecular orbital diagram for c2.
Molecular orbital diagram for he2+. A molecular orbital explicitly describes the spatial distribution of a single electron orbital s, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative ...
Nov 21, 2018 · We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital ...
In addition, for pathway 2, from R2 to product PuN + N, the system passes through a barrier as high as 365.7 kJ/mol (E TS2-E R2), and the length of the Pu1-N2 bond gets progressively shorter, while the length of the N2-N3 bond gets longer and eventually breaks completely.TS1 and TS2 have only one imaginary frequency, which verifies the rationality of the structure.
The molecular energy level diagram for \(\ce{HCl}\) is reproduced in Figure 10.4.5 Figure 10.4.5 : Molecular Orbital Energy-Level Diagram for HCl. The hydrogen 1s atomic orbital interacts molecular orbitals strongly with the 3p z orbital on chlorine, producing a bonding/antibonding pair of molecular orbitals. The other electrons on Cl are best ...
Solved Using The Molecular Orbital Diagram Depicted Below Which Species Have Bond Order Of 3 2p 2p 02p 72p Energy 2s Oa B2 B 02 2 C C22 D N2 Oeco Of Cn G Molecular orbital diagram for b2 . This interaction introduces an element of s p mixing or hybridization into the molecular orbital theory.
8 Jul 2012 — TRANE XL 1200 WIRING DIAGRAM WIRING DIAGRAM - Heating & Cooling question. ... SOURCE: 1994 trane fur…
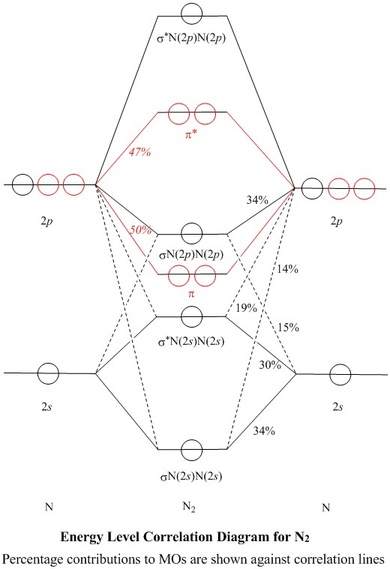
Jul 18, 2014 · The more stabilized 2s orbital does not s-p mix as effectively, due to the greater energy difference between the 2s and 2p orbitals. As nuclear charge increases, s-p mixing becomes less significant. The change of the molecular orbital ordering between nitrogen and oxygen is the manifestation of this decreased s-p mixing.
10 5 Molecular Orbital Theory Chemistry Libretexts . Solved The Molecular Orbital Energy Level Diagram For The . Answer 6 Energy Levels 2a Draw An Energy Level Diagram Cf Tro Fig 48 . Draw The Molecular Orbital Energy Level Diagram For H2 And He2 . Solved Hydrogen Spectrum Energy Level Diagram 120 Draw A
Below is a diagram that shows the probability of finding an electron around the nucleus of a hydrogen atom. Notice that the 1s orbital has the highest probability. This is why the hydrogen atom has an electron configuration of 1s 1. 2) Orbitals are combined when bonds form between atoms in a molecule.
Ionic character in covalent compounds: Bond moment and dipole moment. Percentage ionic character from dipole moment and electronegativity difference. Molecular orbital theory. Molecular orbital diagrams of diatomic and simple polyatomic molecules N2, O2, C2, B2, F2, CO, NO, and their ions; HCl (idea of s-p mixing and orbital interaction to be ...
N2 Molecular Orbital Diagram. With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more familiar diagram. Notice how the σ from the 2p behaves more non-bonding like due to mixing, same with the 2s σ. This also causes a large jump in energy in the 2p σ* orbital.
Molecular orbital diagram of dinitrogen molecule, N 2. There are five bonding orbitals and two antibonding orbitals (marked with an asterisk; orbitals involving the inner 1s electrons not shown), giving a total bond order of three. Atomic nitrogen, also known as active nitrogen, is highly reactive, being a triradical with three unpaired electrons.














.png)


0 Response to "39 molecular orbital diagram for n2 2-"
Post a Comment