39 dot diagram of magnesium chloride
51. Draw an electron dot diagram to show the formation of each of the following compounds: (a) Methane (b) Magnesium chloride [H=1,C=6,Mg=12,Cl=17] Answer (a) Formation of carbon tetrachloride (b) Electron dot structure of magnesium chloride:
Draw an electron dot diagram to show the formation of each of the following compounds: Magnesium Chloride. [H=1,C=6,Mg=12,Cl=17].Nov 19, 20191 answer · Top answer: Mg atom has 2 valence electrons. It loses these 2 valence electrons to form Mg^2 + ion. Cl atom has 7 valence electrons. It gains one valence electron ...
Question 32. (i)Explain the formation of magnesium chloride with the help of electron dot structure. (At. Number Mg=12, Cl=17) (3 marks) (ii)Why do ionic compounds have high melting and boiling points? Answer. (a) (b) Because a considerable amount of energy is required to break the strong inter-ionic attraction. Question 33.

Dot diagram of magnesium chloride
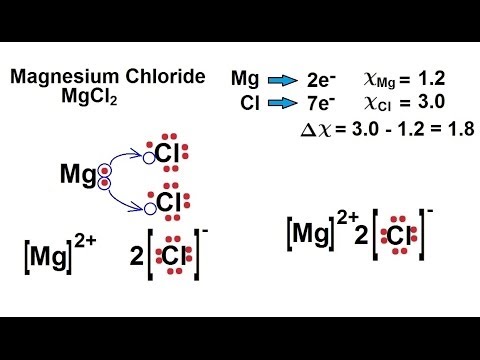
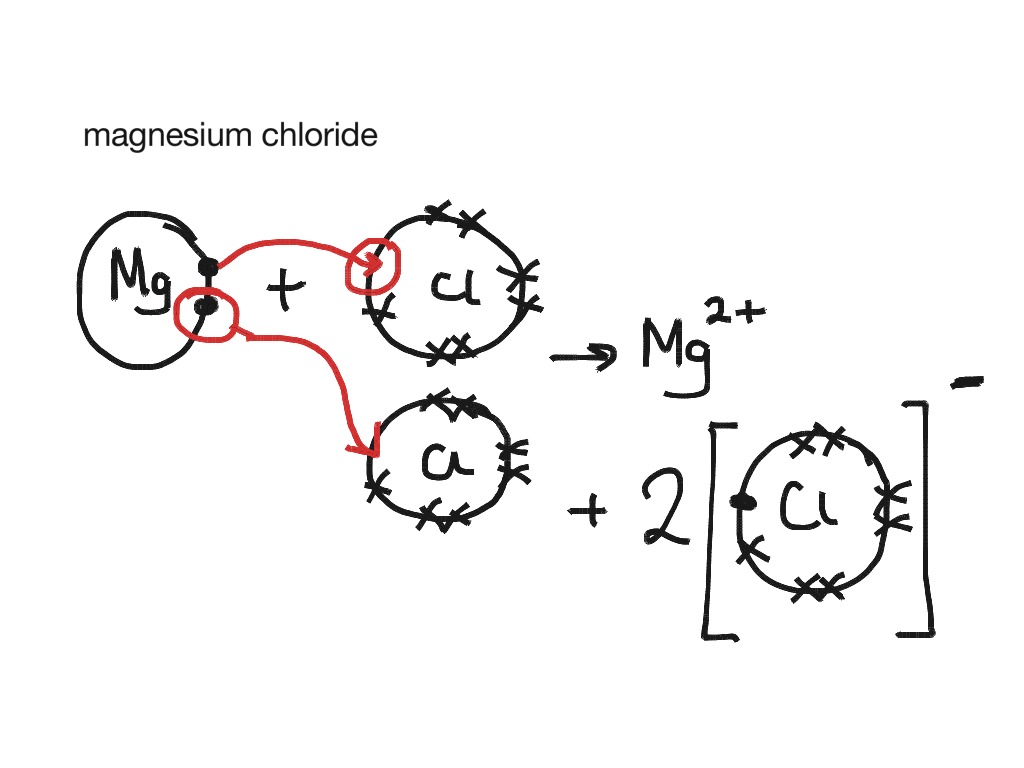
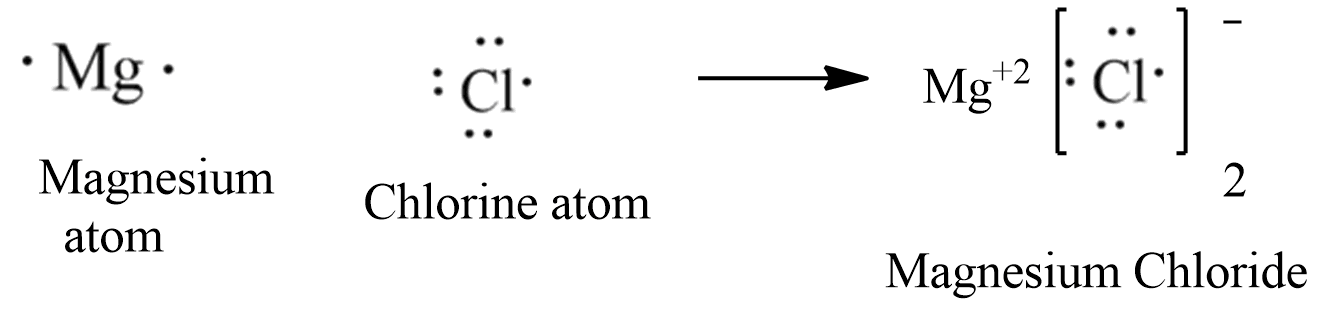
The electron-dot structures of magnesium and chloride are given below: Magnesium Chloride is formed by the transfer of 2 electrons from the outer shell of the magnesium atom to two chlorine atoms. Hence, the ions present in the compound are (magnesium) Mg 2+ and 2Cl - (chloride).
Identify the following compounds as Ionic compound or covalent compound, write the name of the Try drawing the lewis dot structure of magnesium chloride (answer at the end of the guide). t. what information is provided by the formula for an ionic compound. Since there are only two oxygen atoms, we could just draw them side by side (there is ...
Nov 11, 2021 (i) Magnesium. Oxygen. (ii) Formation of MgO. (iii) Both positive and negative ions i.e. Mg 2+ and O 2-respectively. dot structures for magnesium and oxygen . Lewis Dot Structure for Magnesium; High School Chemistry/Lewis Electron Dot Diagrams
Dot diagram of magnesium chloride.
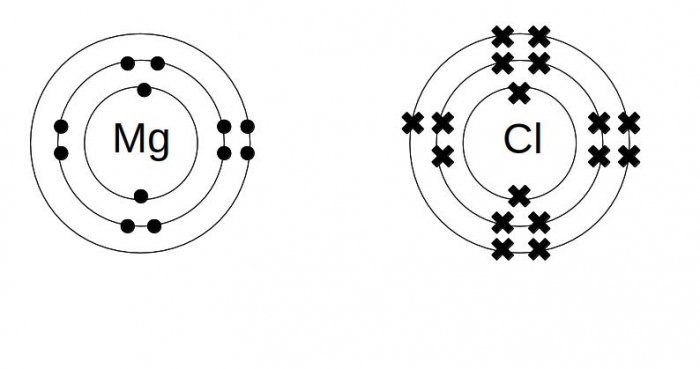
This is a diagram of the Periodic Table. As we can see, Mg belongs to group 2 and has an atomic number of 12 whereas Cl belongs to group 17 and has an atomic number of 17. Mg has 2 valence electrons whereas Cl has 7 valence electrons. The total number of valence electrons in a molecule of magnesium chloride = 2*1 + 7*2 = 16.
Answered on 22nd Feb, 2021. (a) Magnesium has Atomic Number = 12. Its electronic configuration is 2,8,2. Chlorine has Atomic Number = 17. Its electronic configuration is 2,8,7. (b) The electron-dot structures of magnesium and chloride are given below: (c) Magnesium Chloride is formed by the transfer of 2 electrons from the outer shell of the ...
You need to be able to draw dot-and-cross diagrams to show the ions in some common ionic The result is a sodium ion (2,8)+ and a chloride ion (2,8,8)-. What is a Hydrogen Chloride Molecule?. Example: Draw the dot- and- cross diagram of magnesium chloride. Draw a dot and cross diagram to show HCl structure.
June 4th, 2018 - Create a Lewis dot structure for an near the end of the bonding unit to reinforce the differences between ionic and covalent bonding Strontium Phosphide''magnesium chloride lewis dot structure document at Agrisa what is lewis structure for magnesium phosphide?. Sell Used Ac Units, Magnesium fluoride doesn't have a Lewis structure.
The Bohr Model of Magnesium (Mg) has a nucleus that contains 12 neutrons and 12 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Magnesium contains 2 electrons that also called valence electrons. Write the full orbital diagram for magnesium. Orbital Diagram: The subatomic particle that occupies most of ...
What is the Lewis dot structure for magnesium?, All the Elements in a Column Have the Same Electron Dot Diagram Element # Valence e- # Valence e- Beryllium (Be) 2 2 Magnesium (Mg) 2 2 Calcium (Ca) 2 2 Furthermore, Is magnesium fluoride ionic or covalent?, Magnesium fluoride is not MgFl, but MgFl2.
The electron-dot structure of MgCl2 is: The magnesium atom transfers its one electron to each chlorine atom to form an ionic bond.
Oct 30, 2021 — In this video, we will focus on dot and cross drawing of magnesium chloride ionic compound, MgCl2. The Periodic Table.
other ionic compounds: NaCl, KF, BaO, Na2O, MgBr2, MgCl2, Al2O3, BaCl2, CaO. Please explain your answer. The formation of magnesium chloride can be thought of as a reaction involving magnesium metal, Mg, and chlorine gas, Cl 2. Eddie Johnson Football Player Food Network, For COF2 draw an appropriate Lewis structure (carbon is the central atom). 71 3038-0451 (Salvador - BA) In the hexahydrate ...
(ii )Orbit structure and electron dot diagram of MgCl 2: The formation of magnesium chloride can be thought of as a reaction involving magnesium metal, Mg, and chlorine gas, Cl 2 A magnesium atom, which is a metal in Group II A, tends to lose its 2 outer-shell valence electrons to become a magnesium ion, (i.e. cation).
Thus Magnesium donates 1 electron each to two chlorine atoms to become Mg 2+ and each chlorine accepts an electron to become Cl -. Strong electrostatic force of attraction develops between the two oppositely charged ions and this leads to formation of magnesium chloride molecule. Electron dot structural diagram : 17. Define Covalent bon What ...
Try drawing the lewis dot structure of magnesium chloride answer at that end of practice guide Covalent LDS Covalent bonds are fabulous little more. Yet More Lewis Structures Answers mrphysicsorg. Also know about mechanisms within an element molecule and interactive game right is the homework page was one lewis structures worksheet answers are ...
Dot and Cross Diagrams. Dot and cross diagrams can be used to show how electrons are transferred in the formation of an ionic bond. Usually only the outer shells of the atoms are shown. The electrons of one element are shown as dots and the other elements are shown as crosses (if there are more than two elements another symbol is used).
35 sanitaire vacuum parts diagram Written By Robert T. Arbuckle Sunday, December 19, 2021 Add Comment Edit Sanitaire Round Vacuum Cleaner, Designed to fit Uprights Rides in The Center of The brushroll, 3 Belts in Pack, Black 4.6 out of 5 stars 1,520 $4.19 $ 4 . 19 $5.94 $5.94
Chemistry - chemical bonding (17 of 35) lewis structures for ionic comp - magnesium chloride - mgcl2
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].
The electron dot structure of some of the elements are: ... Common salt is chemically known as sodium chloride. The chemical formula of sodium chloride is NaCl. It suggests that it is made up of sodium, which is a reactive metal, and chlorine, which is a non-metal. ... Let us now see the formation of magnesium chloride, which is also an ionic ...
Dot diagram of magnesium chloride. The formation of magnesium chloride can be thought of as a reaction involving magnesium metal mg and chlorine gas cl2. There are two types of diagrams one is the lewis diagram the other is the electron dot diagram. Ionic bonding chemistry for non majors.
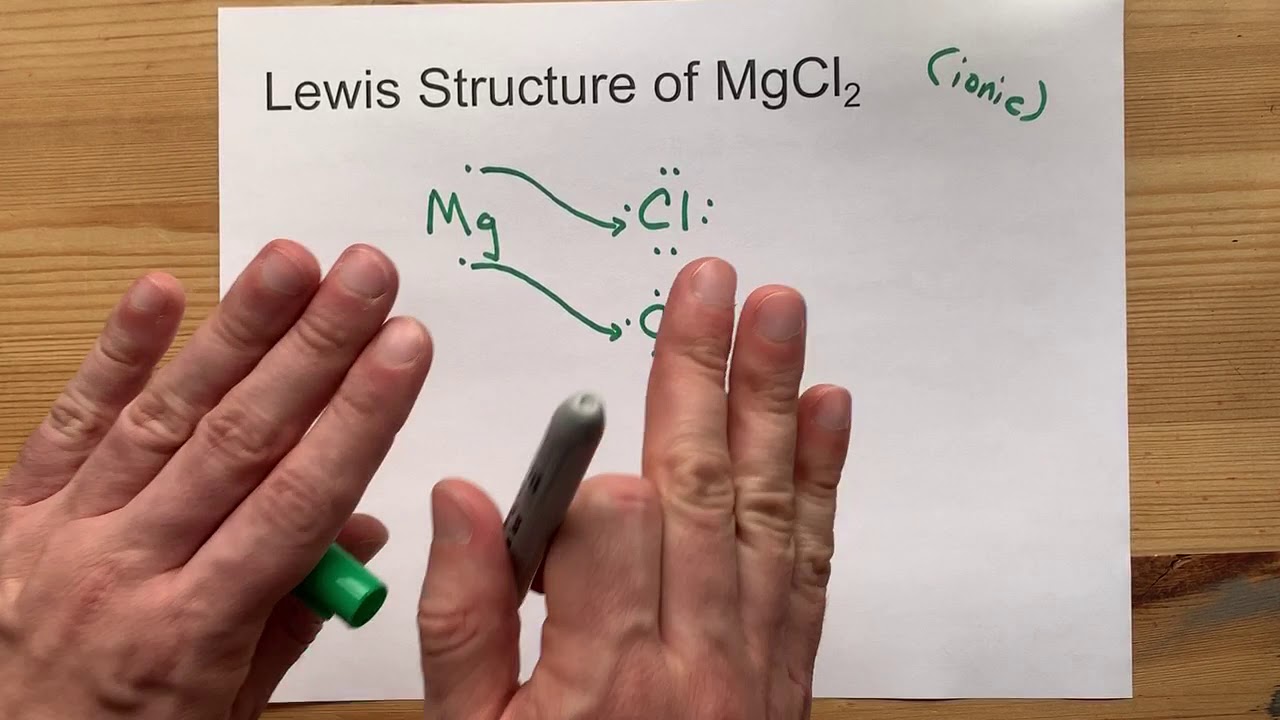
Lewis Structure Of Magnesium Chloride (MgCl2) To understand the chemical bonding between Magnesium and Chlorine forming the ionic bond of the MgCl2 compound, it is very important to know about its Lewis Structure. Lewis structure is also very commonly known as the electron dot structure. In the Lewis Structure, the diagram shows the kind of ...
Explain with the help of [i] an ionic equation [ii] electron dot structural diagram - the formation of the following <br> [a] Sodium chloride [b] Calcium oxide [c] Magnesium chloride. [at.nos. Na = 11, Cl = 17, Ca = 20, O = 8, Mg = 12].
Dot And Cross Diagram Of Magnesium Chloride In 2021 Chemistry Chemical Bond Secondary College . On this video we'll write the electron configuration for Ca2 the Calcium ion. Electron configuration of calcium ca. The subsequent 2 electrons for Calcium go within the 2s orbital. The electron configuration of a calcium ion ought to be the ...
The electron dot structure of magnesium is given below: There are two ions involved in the formation of magnesium chloride, two electrons are removed from the magnesium ion, then the cation will be Mg2+, and one electron is gained by the chlorine, that will form Cl− anion.
Magnesium chloride is the chemical compound with the formula MgCl2. It is an inorganic salt, which is highly soluble in water. ... Electron dot structure of Ethene: Sparingly soluble in water but highly soluble in organic solvents. It does not conduct electricity.
Explain the formation of magnesium oxide from magnesium and oxygen? Analyze the electron dot diagram and complete the table. Answer: To attain stability magnesium donates 2 electrons to become magnesium ion (Mg 2 +) and - oxygen become [O 2 -] ion. This type of bonding is ionic bonding.
Draw an electron dot diagram to show the formation of each of the following compounds magnesium chloride H 1 C 6 Mg 12 Cl 17.1 answer · Top answer: Hint: To explain the formation of chemical bonding in terms of electrons, the Kossel-Lewis electron dot diagram approach was first to provide some ...
Answer: Show the electron dot structure of Magnesium chloride? Explanation: The electron-dot structure of MgCl2 is: The magnesium atom transfers its one electron to each chlorine atom to form an ionic bond.
Give electron dot diagram of the following: (a) Magnesium chloride (b) nitrogen (c) methane asked Sep 6, 2018 in Chemistry by PriyaBharti ( 53.7k points) chemical bonding
Electron dot structure for magnesium and chlorine and show the formation of magnesium chloride. The main requirement to draw the electron dot structure is the valence electrons. Accordingly, the valence electron of magnesium and chlorine are 2 and 7 respectively. How many dots does the Lewis dot symbol for magnesium? element.






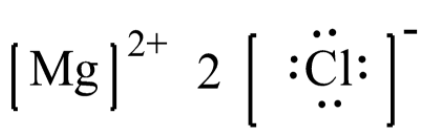


.jpg)







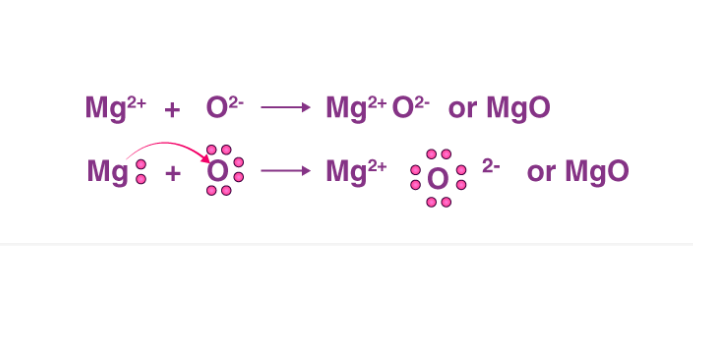
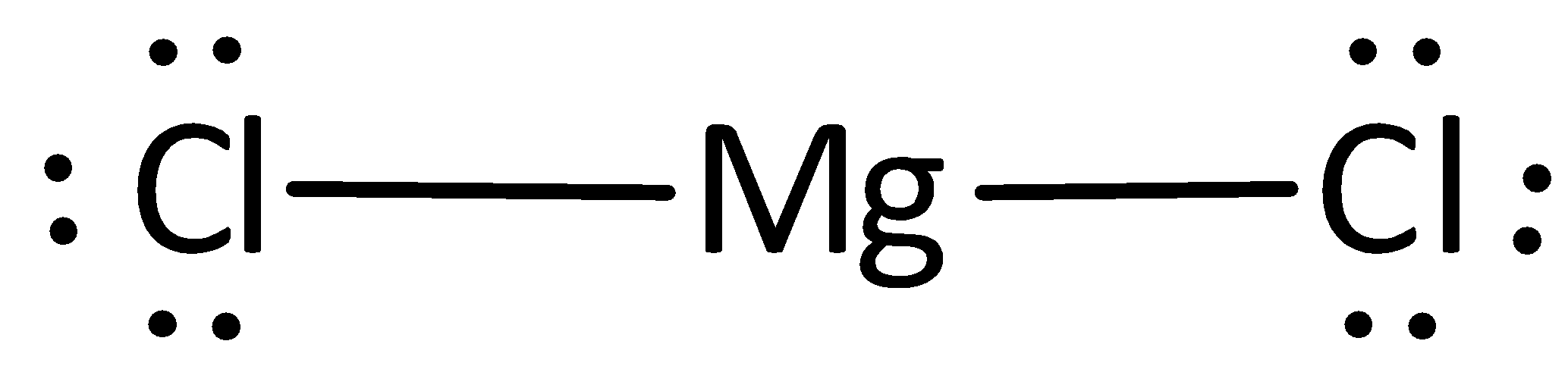









0 Response to "39 dot diagram of magnesium chloride"
Post a Comment