38 bohr diagram for boron
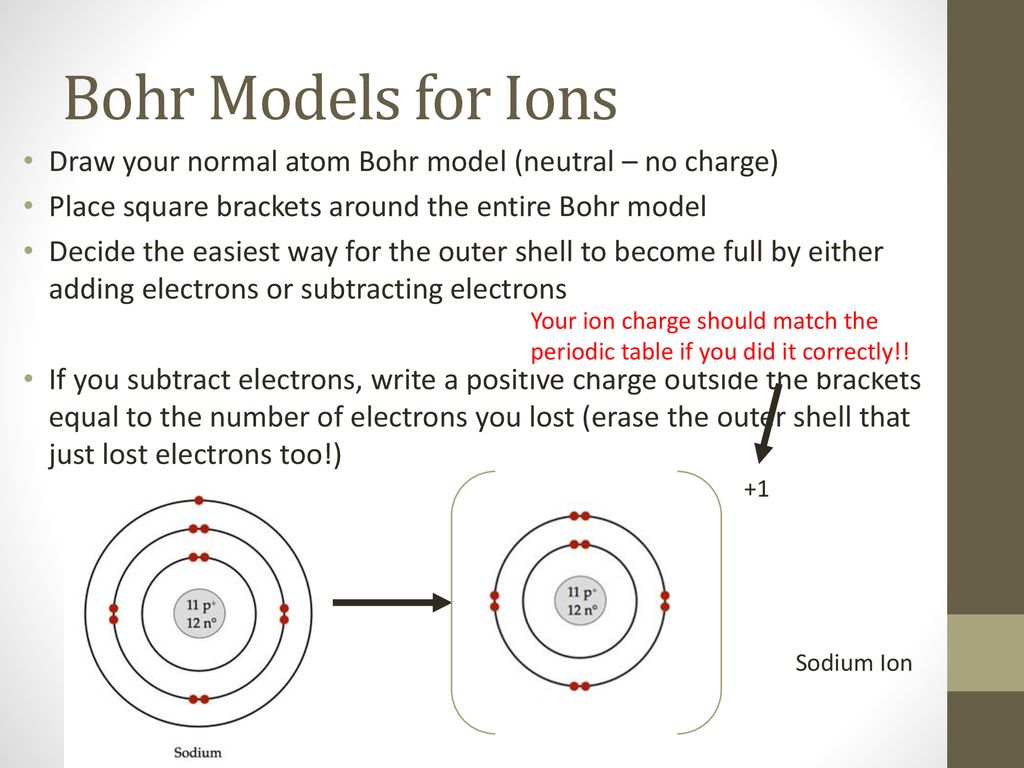
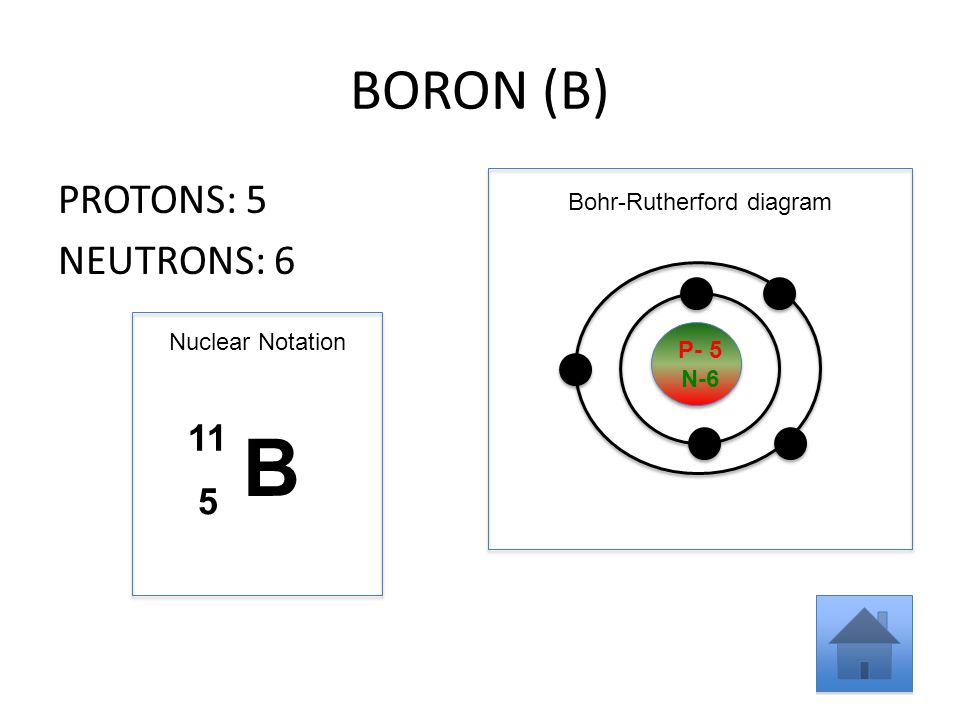
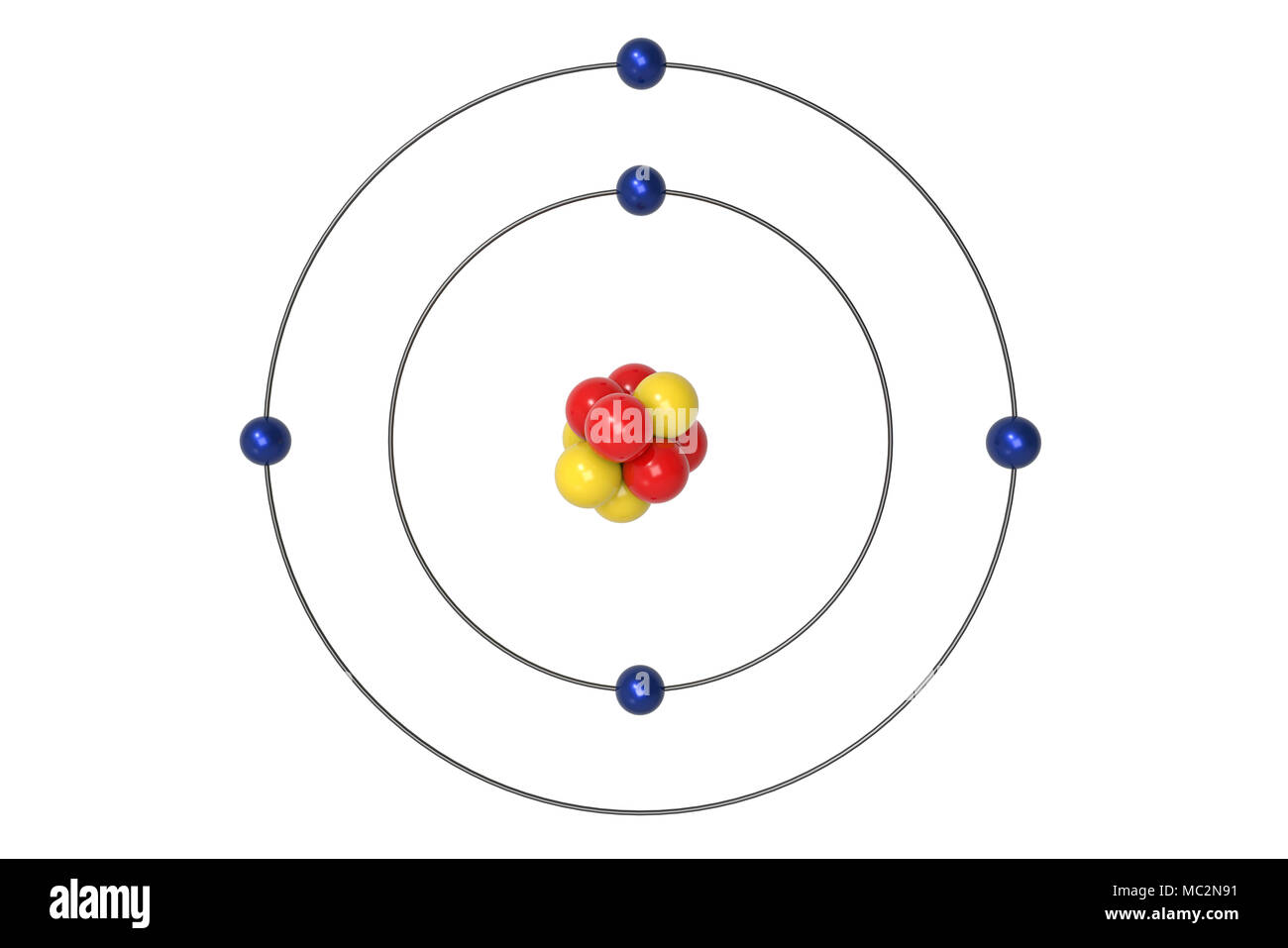
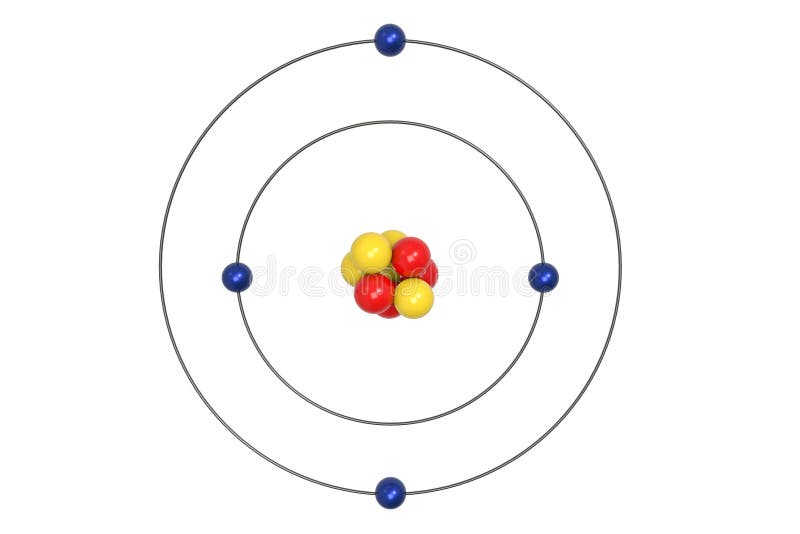
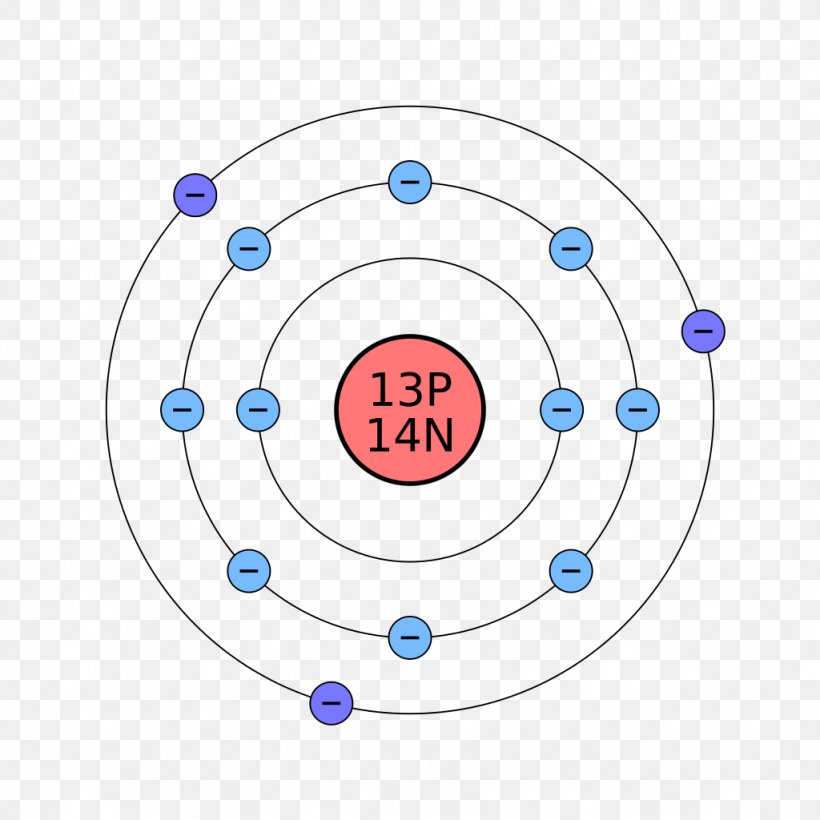
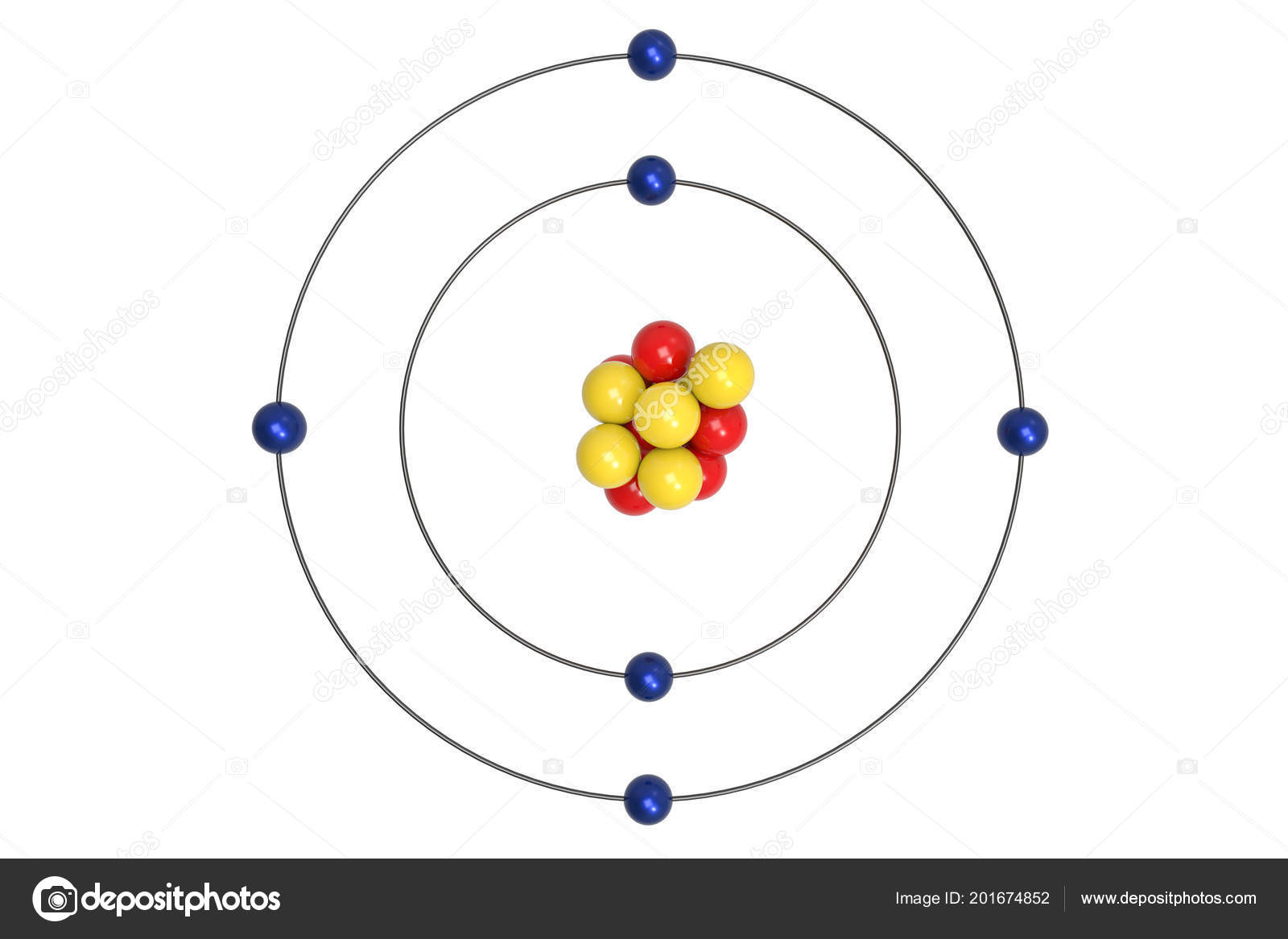
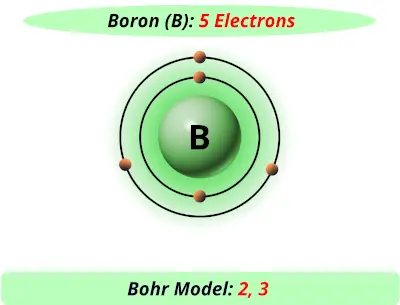
Apr 09, 2018 · A Bohr diagram can be used to visually show the Bohr model of a particular atom. The Bohr diagram for boron shows a central nucleus containing five protons. Bohr Models and. Lewis Dot Structures. Page 2. Bohring. Page 3. Bohr/Lewis Dot Models. Used to predict Draw the Bohr Model for Boron. 1 answerThe Bohr diagram for boron shows a central nucleus containing five protons and neutrons, with its five electrons orbiting the nucleus in two energy...
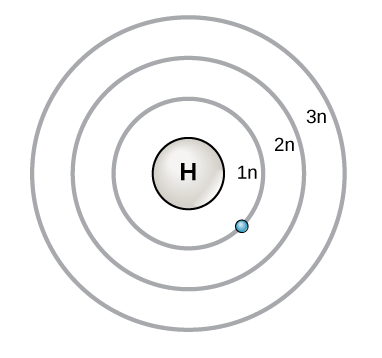
Bohr’s diagram of Boron has only two electron shells (K and L), the inner shell is K-shell and the outermost shell is L-shell. Hence, the electrons found in the L-shell of the Boron atom are its valence electrons because it is the outermost shell that also called the valence shell.

Bohr diagram for boron
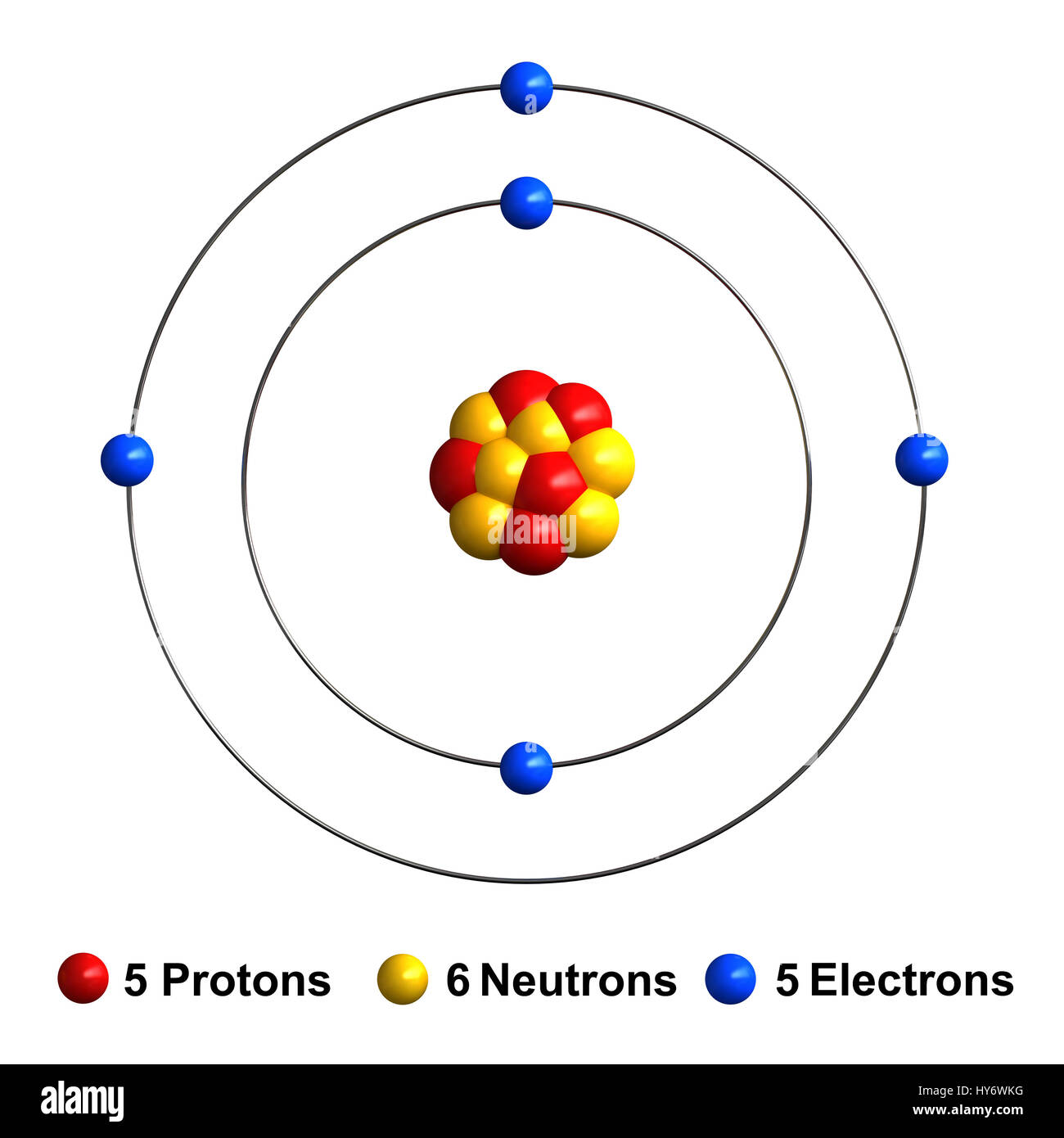
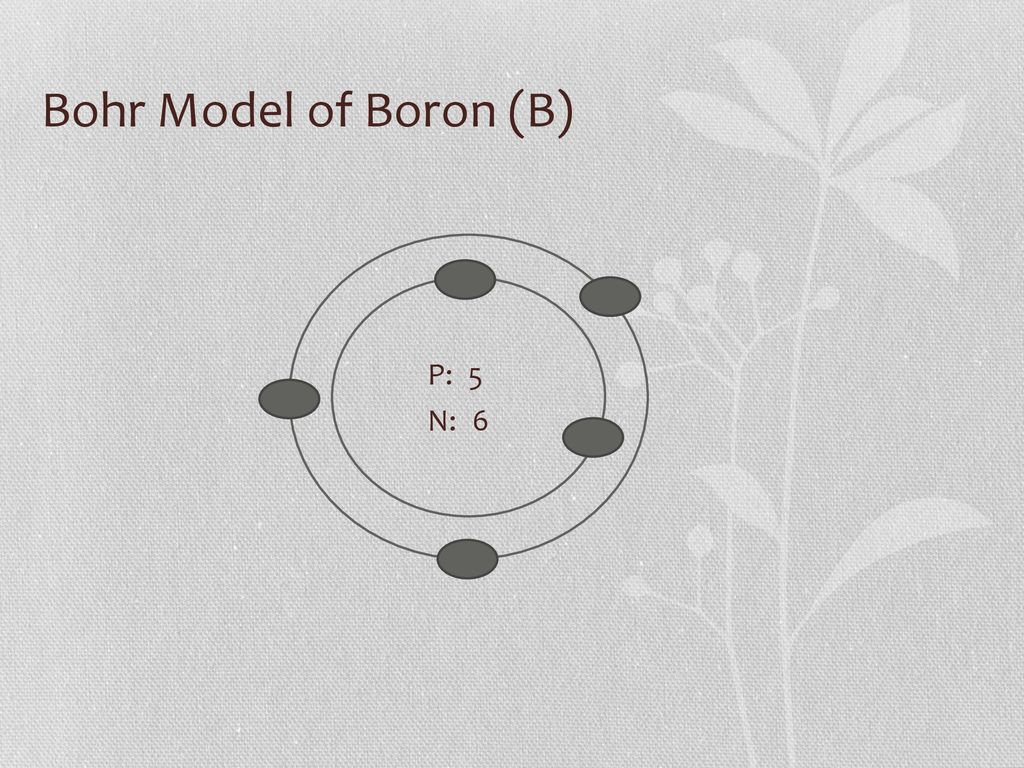
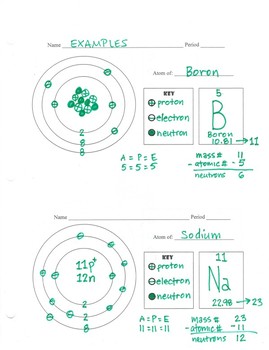
Name: Boron Symbol: B Atomic Number: 5 ... Number of Protons/Electrons: 5. Number of Neutrons: 6 ... [Bohr Model of Boron], Number of Energy Levels: 2. The Bohr model of the boron atom has a nucleus in the center and two energy levels, also known as shells, or orbitals, around the outside. Two electrons orbit ...3 answers · 2 votes: HTo answer this question it is better to understand a little of what the Bohr model is ... For example, a Bohr diagram of the element boron shows five protons and five electrons. A Bohr diagram starts with a simple circle to represent the nucleus, followed by a larger circle around the nucleus to represent the first energy level. An atom's first energy level holds up to two electrons under the Bohr model, represented by two dots.
Bohr diagram for boron. The maximum electron holding capacity in N orbit is 2n2 = 2 × 32 = 32 electrons. The ...28 Jun 2021 · Uploaded by Wayne Breslyn Feb 02, 2021 · The Bohr diagram for boron shows a central nucleus containing five protons and neutrons, with its five electrons orbiting the nucleus in two energy… What pattern do you see between the Bohr models show and their position on the periodic table? There is an obvious pattern to look for: Atomic number increases as you move right along a row. So, using the atomic number of Boron, which is equal to its number of protons, we can determine that Boron has 5 electrons. Now, in the Bohr model of an atom, ... Boron has 2 electrons in its first shell and 3 in its second shell.Check me out: http://www.chemistnate.com
23 Aug 2021 — Bohr's model shows that electrons in atoms are in orbits of different energies around the nucleus (think planets orbiting the sun). Bohr used ...Number of protons: 5Atomic mass: 10,811 atomic mass unitsAtomic number: 5 15 Aug 2020 — Electron Shells. Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons ... For example, a Bohr diagram of the element boron shows five protons and five electrons. A Bohr diagram starts with a simple circle to represent the nucleus, followed by a larger circle around the nucleus to represent the first energy level. An atom's first energy level holds up to two electrons under the Bohr model, represented by two dots. The Bohr model of the boron atom has a nucleus in the center and two energy levels, also known as shells, or orbitals, around the outside. Two electrons orbit ...3 answers · 2 votes: HTo answer this question it is better to understand a little of what the Bohr model is ...
Name: Boron Symbol: B Atomic Number: 5 ... Number of Protons/Electrons: 5. Number of Neutrons: 6 ... [Bohr Model of Boron], Number of Energy Levels: 2.


















0 Response to "38 bohr diagram for boron"
Post a Comment