37 lewis dot diagram n2
May 14, 2021 · The N 2 Lewis structure shows two nitrogen atoms bonded in the same way to each other. If the bond energy for the NN bond is 946 kJmol how much energy is needed to break all the bonds in 30 mol of nitrogen molecules. What is the lewis dot structure for n2. Of system as you could realize these. N2 Lewis Structure Answer.
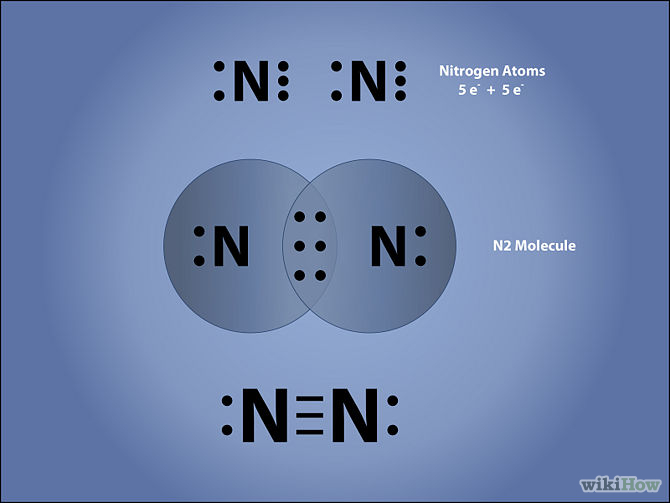
Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell.As per the molecule N2, it has two atoms of Nitrogen.
A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...

Lewis dot diagram n2
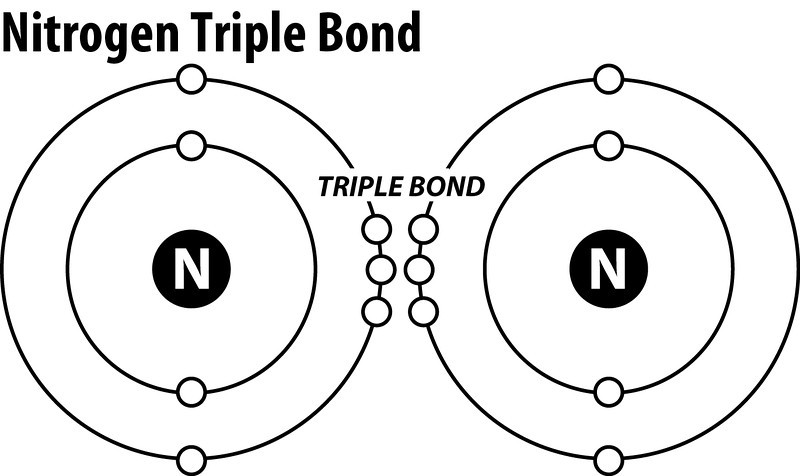
N2 Lewis Structure The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair. N2 Lewis Structure Setup It’s easiest to think in terms of […]
Drawing the Lewis Structure for N 2 (Dinitogen or Nitrogen Gas). Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth's atomsphere is N 2).It also is a good example of a molecule with a triple bond. There are 10 valence electrons available for the Lewis structure for N 2.. Video: Drawing the Lewis Structure for N 2
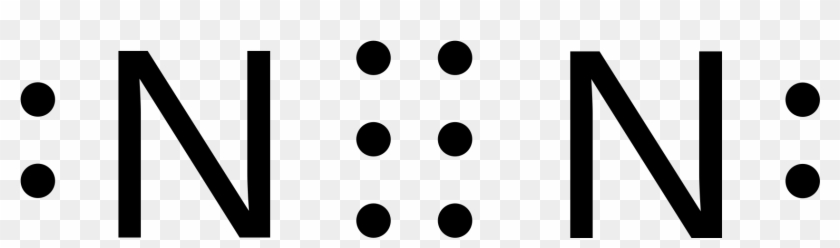
The lewis dot diagram for an N2 molecule is :N:::N: . What is the dot diagram for Kr? Kr is a noble gas, so its dot diagram will feature eight valence electrons surrounding the Kr symbol, in four ...
Lewis dot diagram n2.
9. Draw a Lewis dot diagram for the barium atom. 10. Draw the Lewis dot diagram for the silicon atom. I I, Draw the Lewis dot diagram for the iodine atom. 12. Draw the Lewis dot diagram for the xenon atom. 13. Hypothesize: Why are noble gases considered to be non-reactive? Your group will check your answers with the instructor before moving on.
Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice. Lewis Structures are important to learn because they help us predict: the shape of a molecule. how the molecule might react with other molecules. the physical properties of the molecule (like boiling point, surface tension, etc.).
How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com
Nov 19, 2018 · The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter N's, so that there would be 6 dots total What is the Lewis dot structure for N2 look like?
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The total number of valence electrons between the two N atoms is 10 e^-.
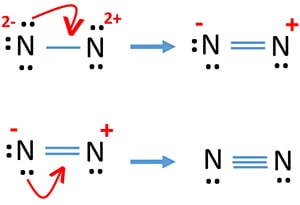
Based on the Lewis electron-dot diagrams of N2 and N2H4, N2 has a stronger nitrogen-to-nitrogen bond than N2H4. The strength of a bond is dependent on the bond length and the bond order. The higher the bond order, the shorter and stronger the bond. Hence triple bonds are stronger than double bonds and double bonds are stronger than single bonds.
Nov 17, 2021 · Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen.
A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons.
N2 dot structure would comprise of two atoms of Nitrogen(N) atoms. There is a triple bond between both nitrogen atoms. Each N is surrounded by two dots which are called lone pairs of electrons. It is a diatomic nonpolar molecule with bond angles of 180 degrees. 6. Explain o2 lewis structure in simplest form. Two oxygen atoms are joined by a ...
Problem Details. Write Lewis structure of N2. Q. The structure of borazine, B3N3H6, is a six-membered ring of alternating B and N atoms. There is one H atom bonded to each B and to each N atom. The m... Q. The structure of borazine, B3N3H6, is a six-membered ring of alternating B and N atoms. There is one H atom bonded to each B and to each N atom.
Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each other, and then we'll put two valence electrons between them to form a chemical bond.
Lewis dot diagram for n2. 25 it has five electrons in its outermost valence shell. If you are talking about the lewis dot diagram then n 2 would have 5 dots around each of the letter ns so that there would be 6 dots total what is the lewis dot structure for n2 look like. The lewis dot structure of a nitrogen atom would be the capitol letter n ...
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
Answer (1 of 3): Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple covalent bonds. The remaining lone pairs are repulsed by this dense electronegativity and so are drawn to appear as far away as possible.
In the Lewis structure of the N2 molecule, over there is a formation of a triple covalent bond stood for by three lines between two atom of Nitrogen. The leftover 2 2p orbitals become two π bonds and electrons making a pair in between the nitrogen atoms will make a sigma bond.
The Lewis dot structure for Nitrogen will be this: However, when two atoms of Nitrogen bind together, it has the following structure: Here both these atoms share six valence electrons to form a triple bond. These electrons are shared equally, and both Nitrogen atoms have one lone pair of electrons. N2 Polarity
Answer (1 of 2): In exactly the same way as you'd do it for any simple, covalently bonded compound. First you need to know the valency of each element. Then you need to draw a stick model with bonds between the atoms, so that each atom's valency is satisfied (so N has three bonds coming out of i...
What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable.
The dot structure of Na+1 is Na+1 . The dot structure of O-2 is O-2. Note that Na is in group 1 and should lose 1 electron while O is in group 6 and should gain 2 electrons. ionic compounds Make certain it's ionic: one atom must be from groups 1-3, the other from groups 4-7 (including H).
Answer (1 of 2): In exactly the same way as you’d do it for any simple, covalently bonded compound. First you need to know the valency of each element. Then you need to draw a stick model with bonds between the atoms, so that each atom’s valency is satisfied (so N has three bonds coming out of i...
Lewis dot diagram for n2. Each nitrogen atom also has a lone pair of electrons. The remaining lone pairs are repulsed by this dense electronegativity and so are drawn to appear as far away as possible. The lewis dot structure for any molecule can be found by following a general set of rules consisting of 5 or sometimes 6 steps.
Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2.
NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.
11+ N2 Lewis Structure. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs ) and one shared pair of electrons (written between the atoms). Calculate the total valence electrons in no2 molecule. Nitrogen Fixing Plants to Fertilize…















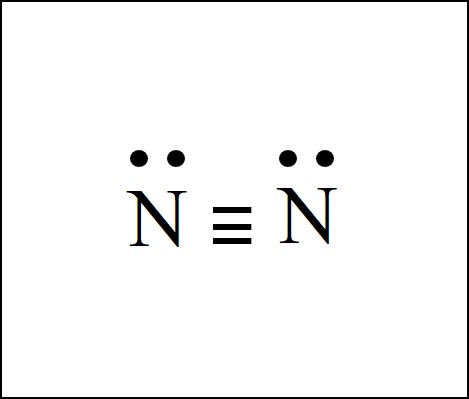




![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://d1hj4to4g9ba46.cloudfront.net/questions/1890007_1909574_ans_16e2a124f2974b5694de1a9f3c97eebd.png)




0 Response to "37 lewis dot diagram n2"
Post a Comment