37 lewis dot diagram for so3
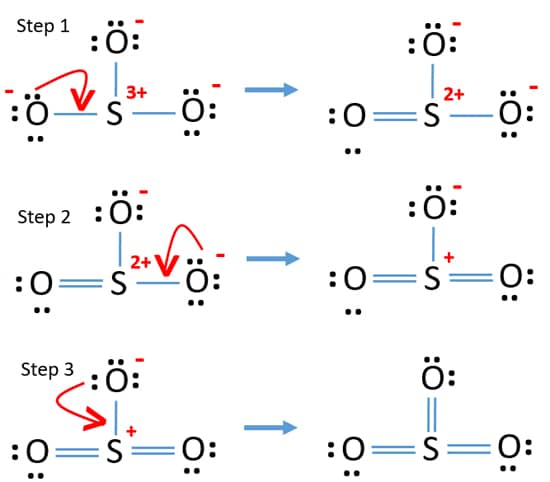
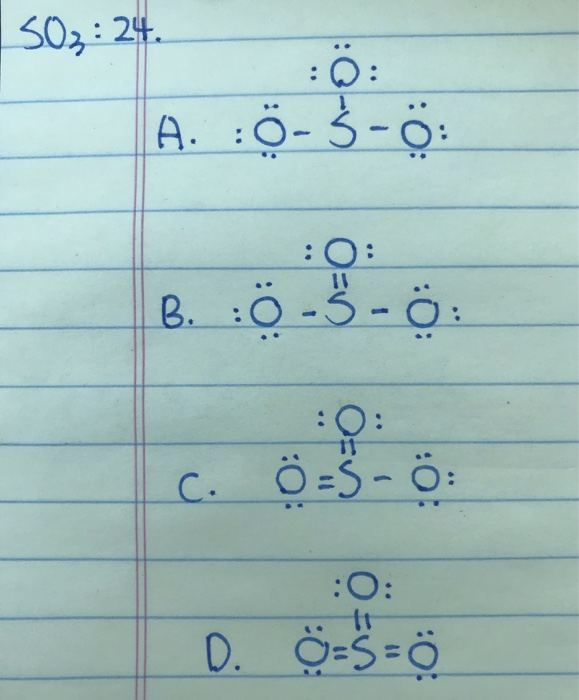
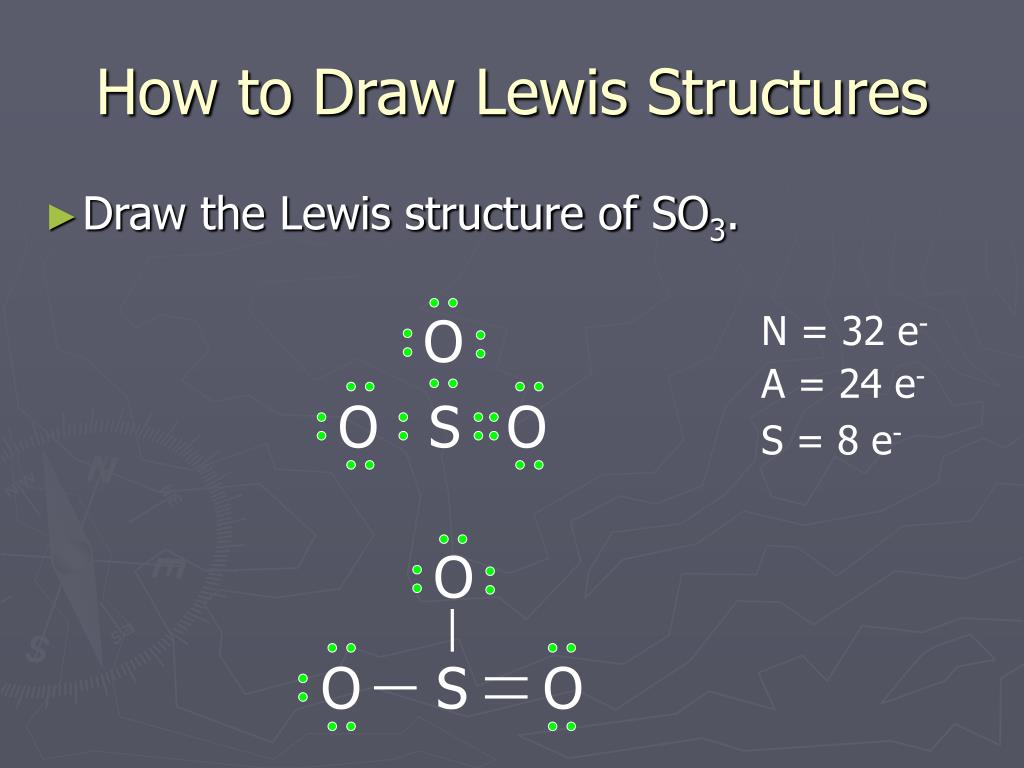
A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number... Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure for SO 3 .
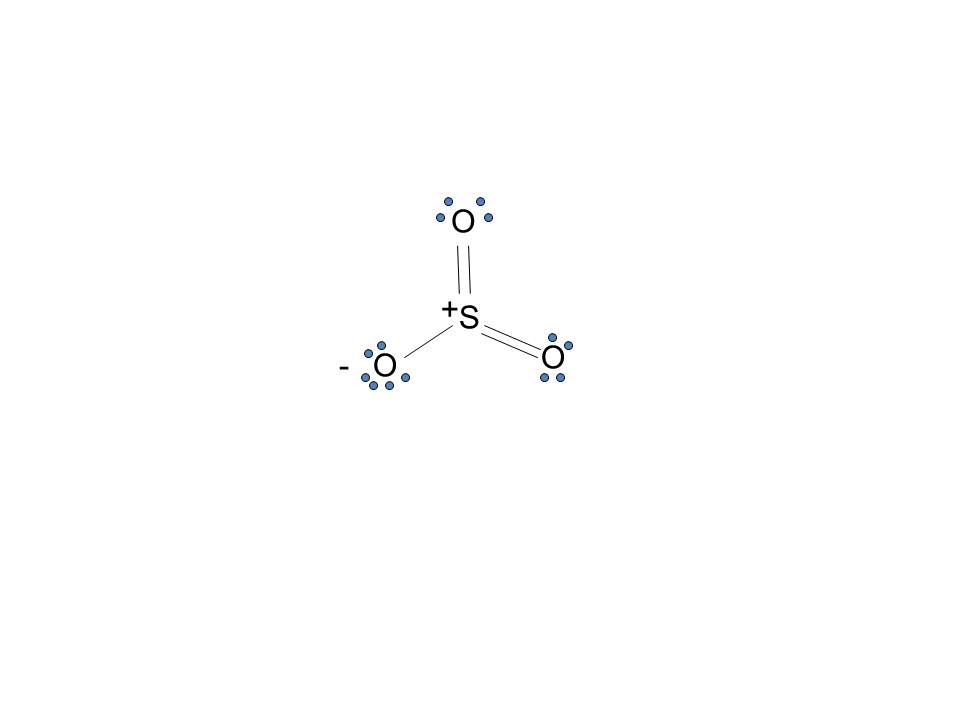
simple procedure for drawing the SO3 lewis structure, so3 lewis electron dot structure, electrostatic potential, ESP, resonance structures of so3, Lewis structures of SO3| Lewis configuration of sulfur trioxide SO3, octet rule, SO3 Lewis structure, π bonds, 2π electrons, multiple bonds, stable resonance, resonance structures, so3 resonance or double bonds, lewis structures and the octet ...

Lewis dot diagram for so3
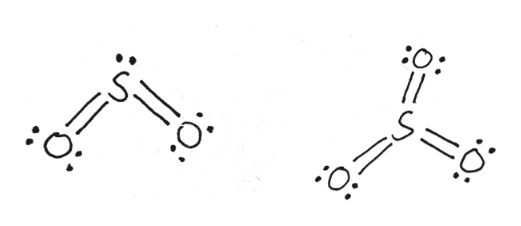
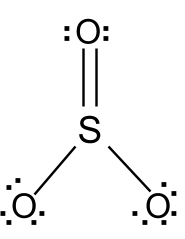
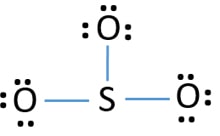
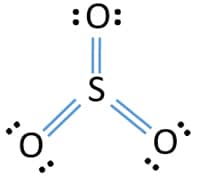
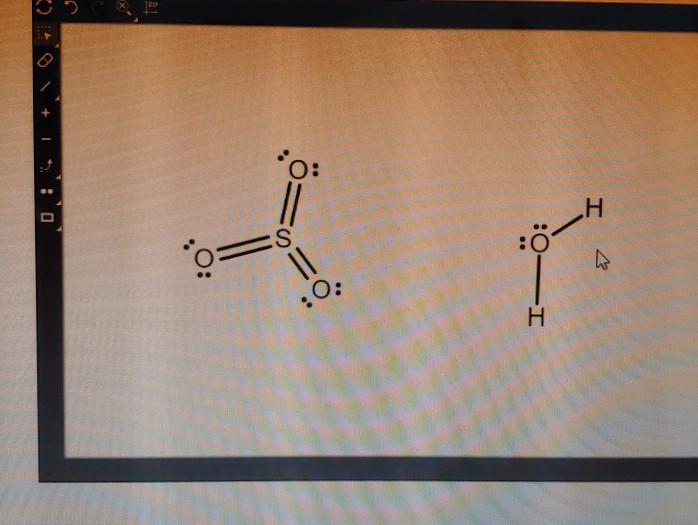
In the SO3 Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the SO3 Lewis structure, with all three oxygen atoms arranged in trigonal planar geometry. Add valence electrons around the oxygen atom, as given in the figure. Step-3: Lewis dot Structure for SO3 generated from step-1 and step-2 Lewis dot diagram for so3. Lets do the so3 2 lewis structure. So we can write the formal charge for sulfur as zero. For the so3 2 compound we have 26 total valence electrons and that includes these two electrons up here there are two extra valence electrons. It is a form of pollution. So we have 26. This problem has been solved! Write a single Lewis structure for SO3. Draw the Lewis dot structure for SO3. Include all lone pairs of electrons. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
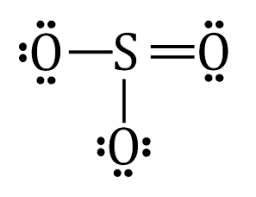
Lewis dot diagram for so3. A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion). For the SO3 2- Lewis structure the total number of valence electrons ... SO3 Molecular Geometry, Lewis Structure, and Polarity Explained. SO3 stands for Sulfur Trioxide. This is one of the most pollutant chemical compounds in the gaseous form. It is also a primary agent in the acid rain. The main use of this component is to make sulfuric acid for industrial purposes. How to draw the Lewis Structure of SO3 (sulfur trioxide) - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electrons!Check ... Dec 02, 2021 · The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. The Lewis structure of sulfur trioxide (SO3) molecule is drawn by: First, look for the total number of valence electrons in a single sulfur trioxide (SO3) molecule, which is twenty-four.
This chemistry video tutorial explains how to draw the lewis structure of SO3 also known as Sulfur Trioxide. It discusses the molecular geometry, bond angle... Let's do the SO3 Lewis structure. Sulfur has 6 valence electrons. Oxygen has 6, but we've got three Oxygens, for a total of; 6 plus 18; 24 valence electrons. Let's put Sulfur at the center and then the Oxygens around the outside, all three of them. Now we'll put two valence electrons between each atom to form a chemical bond. We've used 6. This problem has been solved! Write a single Lewis structure for SO3. Draw the Lewis dot structure for SO3. Include all lone pairs of electrons. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Lewis dot diagram for so3. Lets do the so3 2 lewis structure. So we can write the formal charge for sulfur as zero. For the so3 2 compound we have 26 total valence electrons and that includes these two electrons up here there are two extra valence electrons. It is a form of pollution. So we have 26.
In the SO3 Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the SO3 Lewis structure, with all three oxygen atoms arranged in trigonal planar geometry. Add valence electrons around the oxygen atom, as given in the figure. Step-3: Lewis dot Structure for SO3 generated from step-1 and step-2










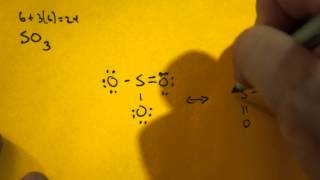





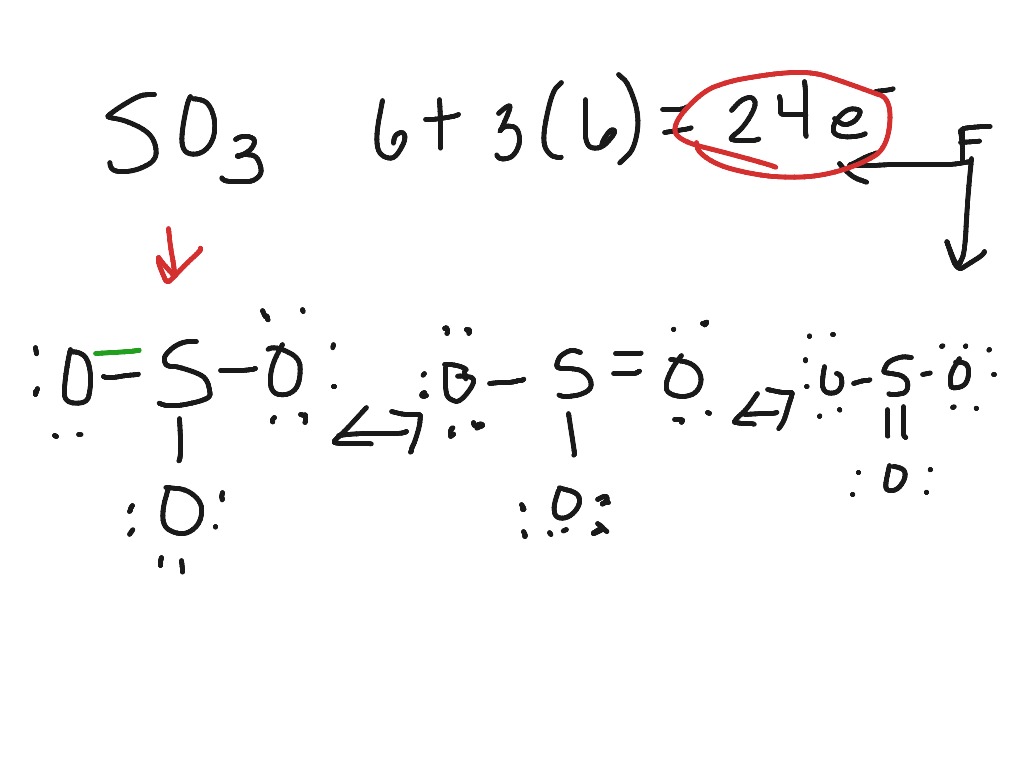





0 Response to "37 lewis dot diagram for so3"
Post a Comment