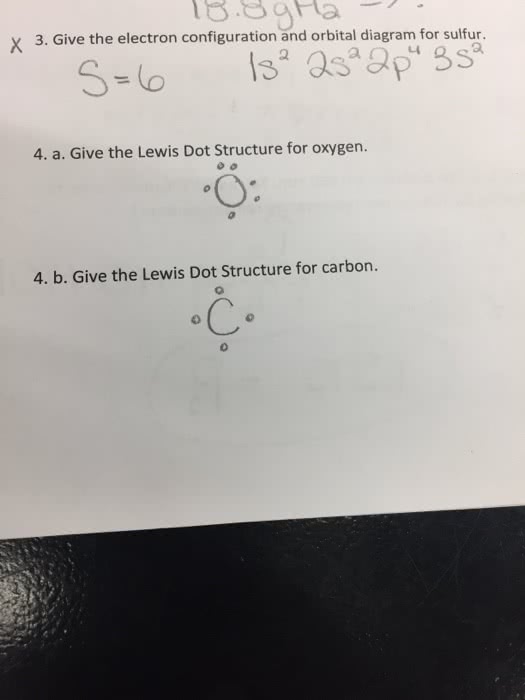
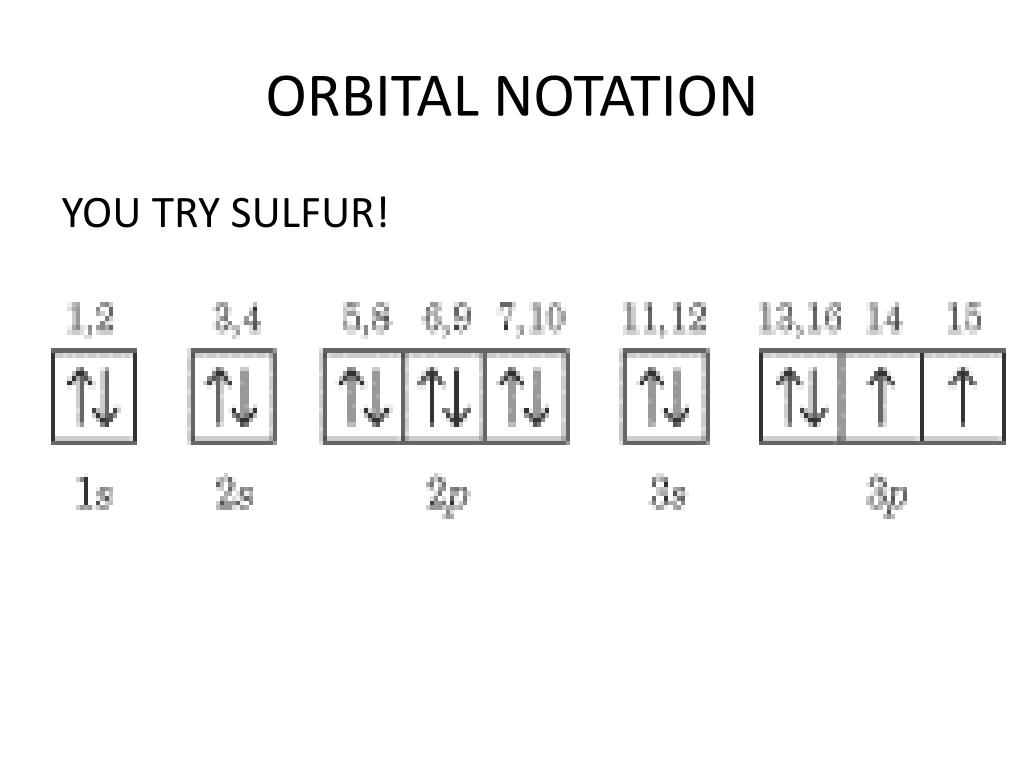
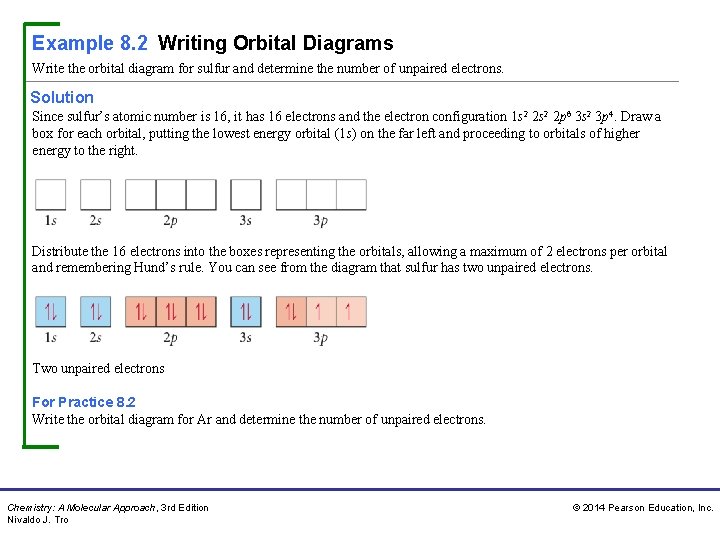
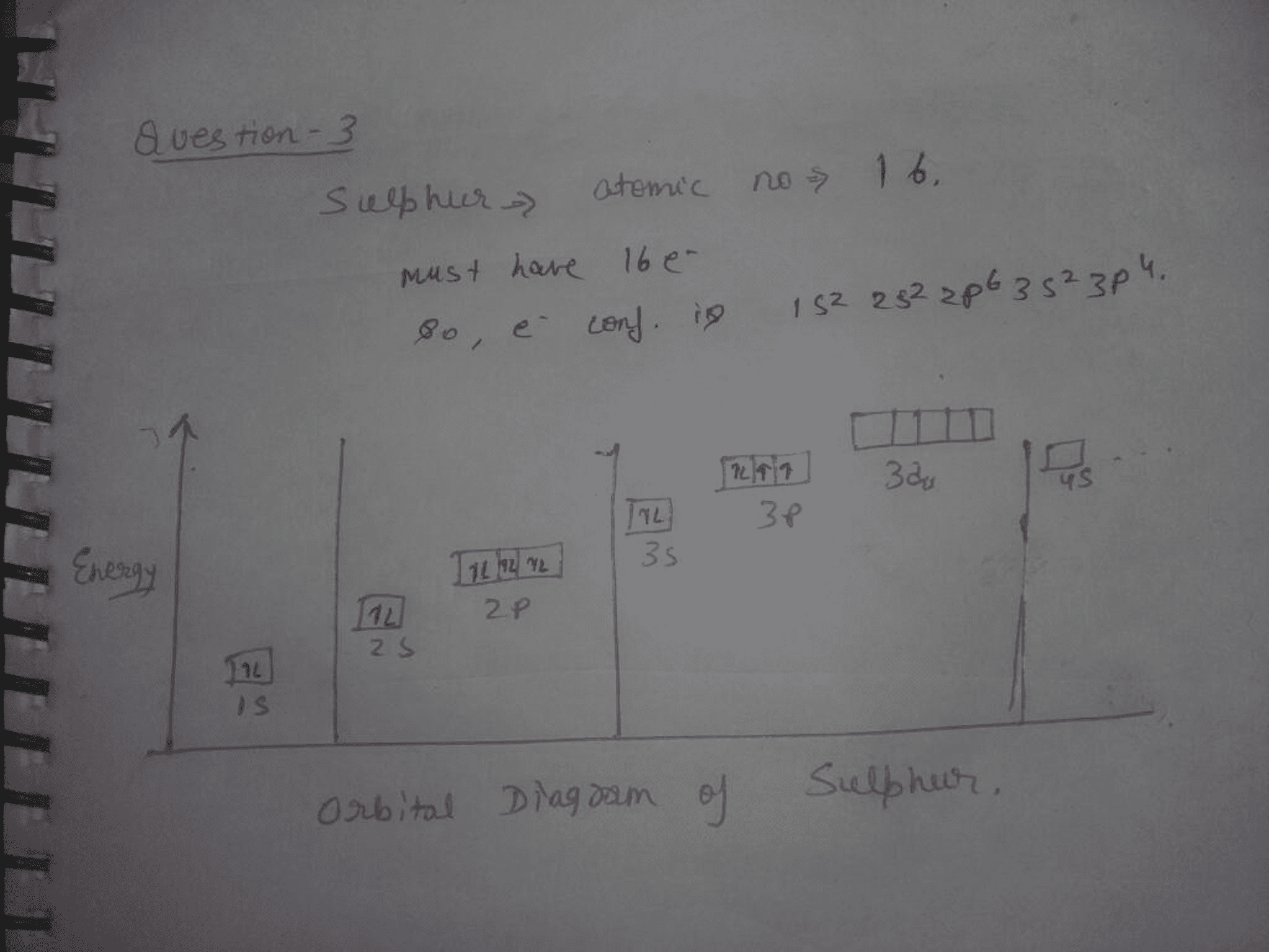
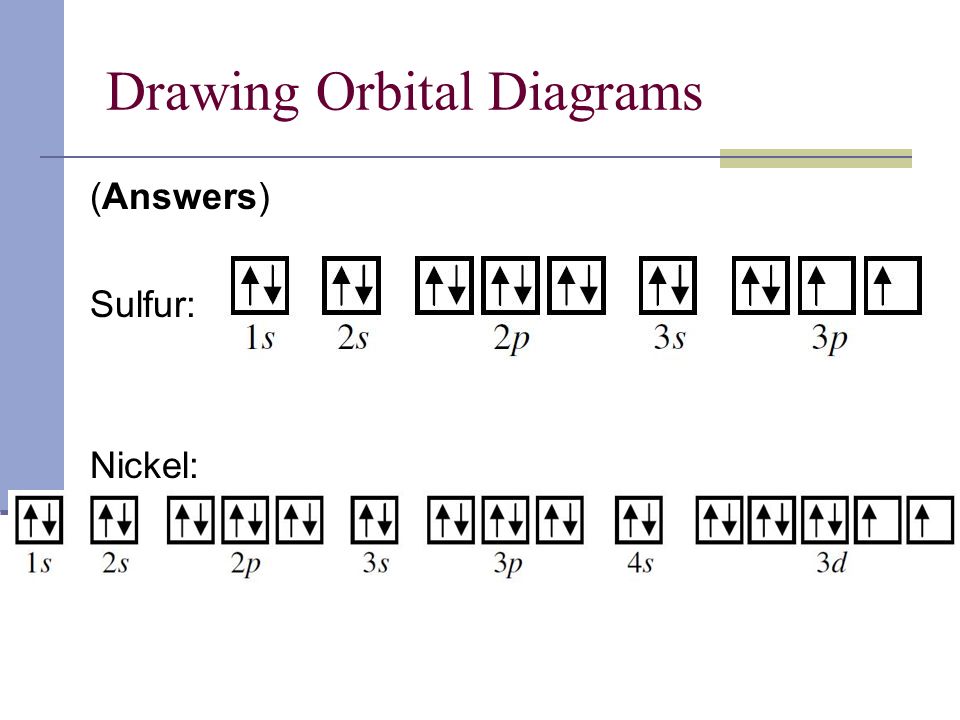
36 orbital diagram for sulfur
When we draw the Lewis structure, the best structure is the one where S has the negative formal charge and each fluorine is singly bonded to the sulfur. So in my tutorial question, we are asked to draw the valence orbital diagram for F, S, S-, S*- and S hyb. So for F and S it's just the normal case. But for S-, isn't this assuming that sulfur has the negative ionic charge? I thought all it has is a negative formal charge where the formal charge does not indicate the presence of a negative ioni... 1 answerSulfur is an element that belongs to the p-block. The atomic number of sulfur is 16, so contains 16 electrons arranged in increasing order of energy ...
So I need someone to check some of these, so I might crosspost this to other subreddits, if you know any, please do. Or if you are an expert yourself, please correct me if there's any mistakes. But I did watch Dr. Stone in an *Anime Streaming website*, I posted some interesting comments in the discussions of Dr. Stone Episodes. I will post them in a chronological order with the matching episodes. Although, I think it's a bad idea to post this in a whole one post. Because no one gonna read it t...

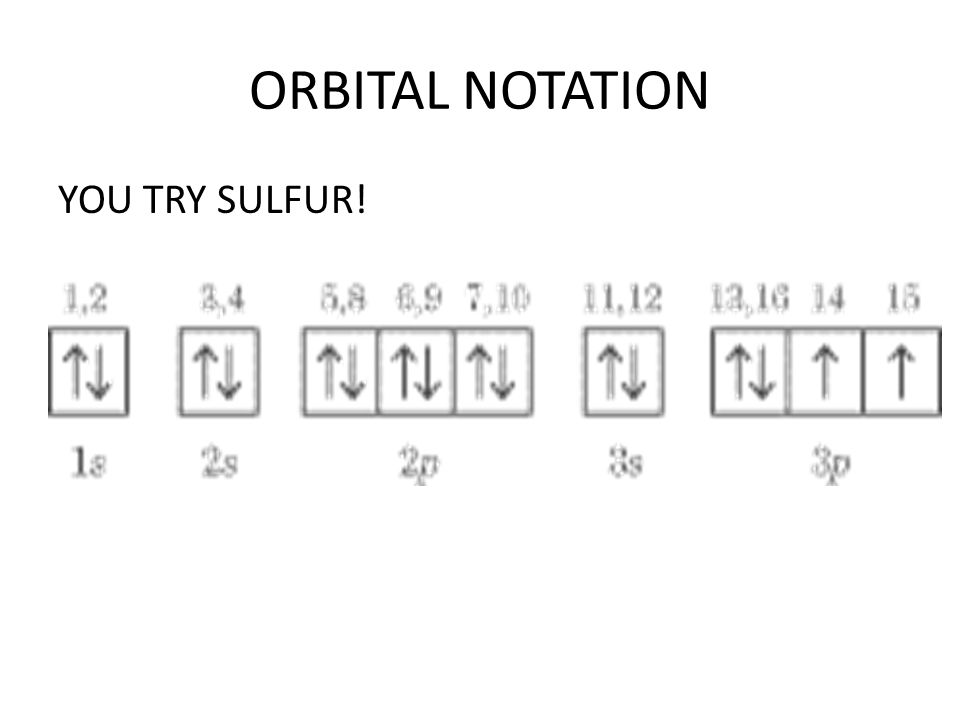
Orbital diagram for sulfur
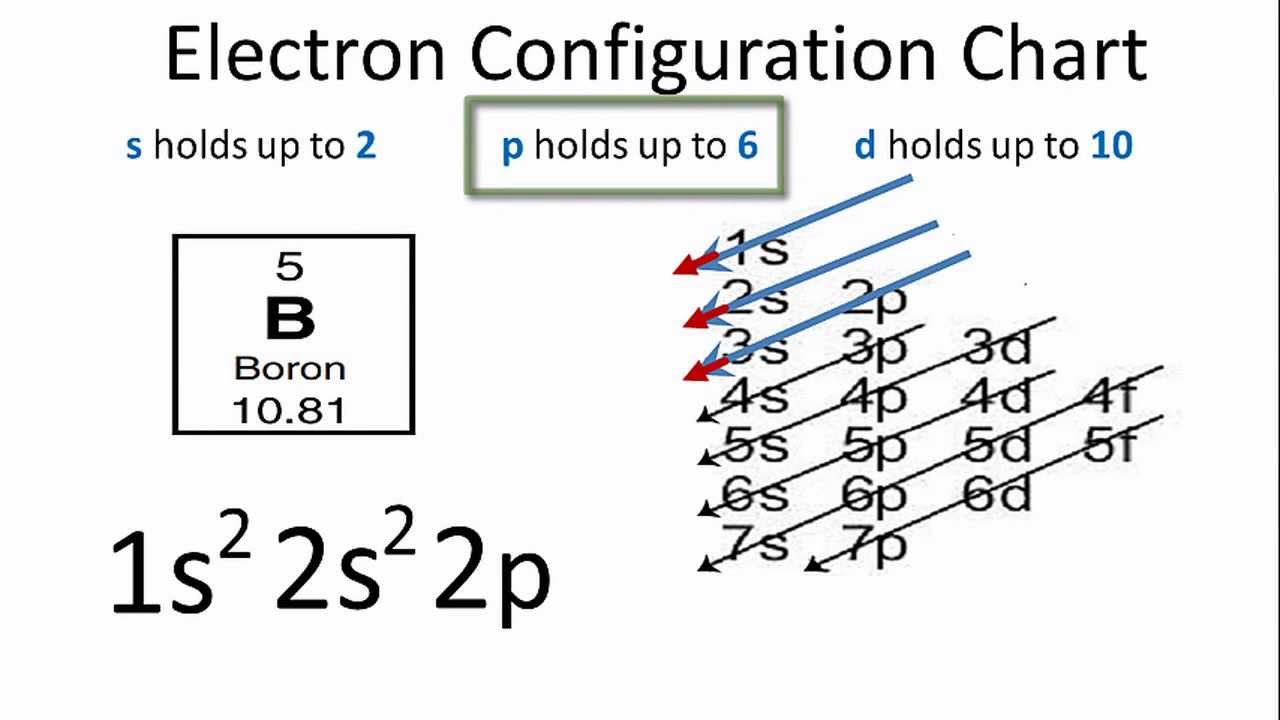
Sulfur (S) has an atomic mass of 16. Find out about its chemical and physical ... Electron Configuration, [Ne] 3s2 3p4. 1s2 2s2 2p6 3s2 3p4. Orbital Diagram. Jan 26, 2021 — when we the electron configuration of Sulfur the first two electrons go in the 1s orbital. As 1s only hold two electrons and the next two ... [Original post I made in /r/Astronomy](https://www.reddit.com/r/Astronomy/comments/3fda00/atmospheres_from_stars_to_planets_everything_you/) Hey, /r/space! I recently answered a [question](https://www.reddit.com/r/askscience/comments/3esx20/how_does_pluto_sustain_any_kind_of_atmosphere/) on /r/AskScience about how Pluto can sustain an atmosphere while the Moon does not. This got me thinking that perhaps people would like to know more about atmospheres as an astronomical phenomenon in genera...
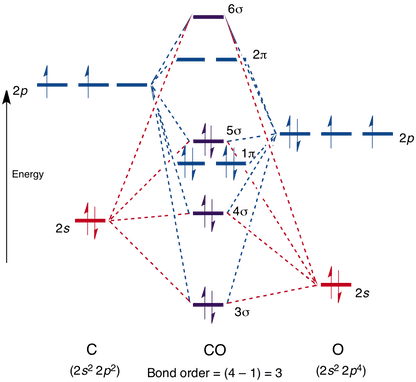
Orbital diagram for sulfur. Hey, /r/astronomy! I recently answered a [question](https://www.reddit.com/r/askscience/comments/3esx20/how_does_pluto_sustain_any_kind_of_atmosphere/) on /r/AskScience about how Pluto can sustain an atmosphere while the Moon does not. This got me thinking that perhaps people would like to know more about atmospheres as an astronomical phenomenon in general. As such I have decided to write a post which will talk about atmospheres of stars and planets in general as well as the atmospheres in our ... I am doing an exercise about the MO diagram for SF4. For sulfur, the 2s orbital and one of the P orbitals have the A1 Mulliken symbol, and 2 of my SALCs for F have the A1 Mulliken symbol as well. I know that I will get one bonding and one antibonding MO from their interaction, but would the other two A1 MOs be nonbonding, or would one be bonding and one antibonding? If I make it so out of my four A1 MOs, two are bonding and two are antibonding, I get a bond order of 4, which makes sense for... Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra... Draw a fully labelled molecular orbital energy level diagram for the molecule SCl. Show only the molecular orbitals made from the sulfur 3p orbitals and the chlorine 3p orbitals (you can use boxes or lines to indicate orbital energy levels). Label the molecular orbitals as σ or π and indicate whether they are bonding or antibonding orbitals. You can assume that the 3p S atomic orbitals are of slightly higher energy than the 3p Cl atomic orbitals in your diagram. i.Calculate the bond order for S...
# It's Hive, so I'm sure it's going to be strange. But at its core, their "magic" is still just science[.](https://bungie.net/common/destiny_content/grimoire/hr_images/603040_865612de633ac3e8a3c8c793f6fd0122.jpg) – [Ana Bray](https://www.ishtar-collective.net/transcripts/adventure-deathly-tremors) I’ve wanted to make this post for some time now and have been considering the science of Hive Magic since Stasis was revealed as a Darkness subclass. A while ago I read the post “[Hive soulfire as a p... Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra... What would the orbital diagram for these molecules look like if they had this many electrons? ​ I'd guessed that phosphorus would have 3s(2), 3p(3), and 3d(5). The half-filled p and d orbitals would offer stability to explain why phosphorus can sometimes make 5 bonds. Alternatively I was considering 3s(2), 3p(6), and 4s(2). ​ I'd guessed that sulfur would have 3s(2), 3p(3), 3d(5), and 4s(2). Again, the half-filled p and d orbitals would offer stability. An electron from... **“Sure, go ahead - try to keep information from getting free. See how that turns out.”** **-Unsigned journal entry at a tenth age laboratory dedicated to reverse-engineering Ktarebte-Blackbottom subreal matrices, recovered from the remains of the facility’s annihilation** “I can’t decide if I’m going to give you the highest of accolades or murder you.” The woman dropped the leather sleeve of tools, glaring around the - in the most non-metaphorical sense possible - palatial room. It held a...
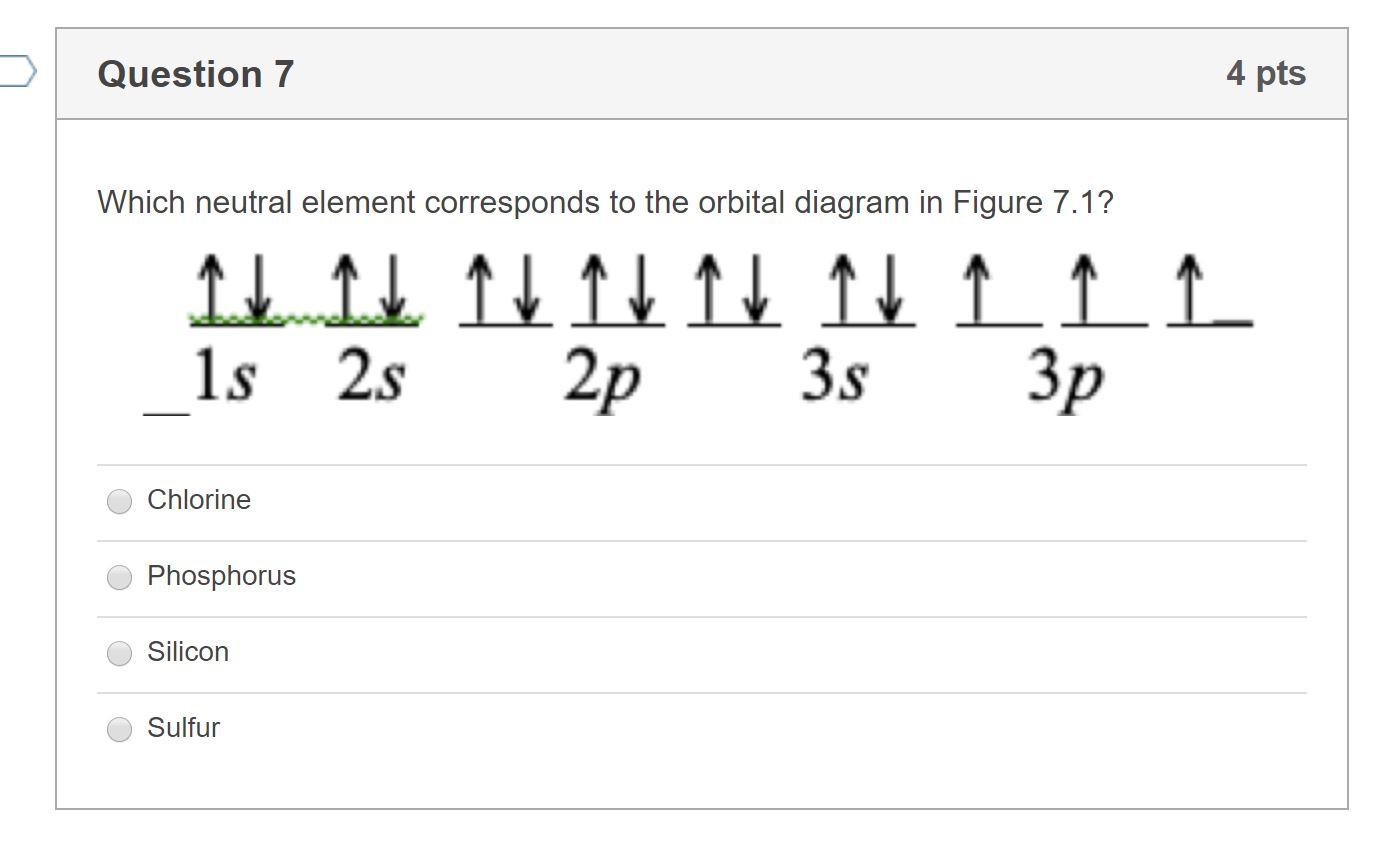
So I'm making this post for a few reasons. Personally, I want all of this off of my laptop and in a place I can easily get to on my phone to argue with people about climate change. The reason I'm posting it here is because I want Steven to have enough information to confidently confront climate change deniers and do the factual equivalent of a curb stomp from orbit, preferably on stream for my entertainment. Let's get riiiiiiiiiiiiiiight INTO THE MEMES! #Evidence of Climate Change Here is ev... >I'm keeping the original post in tact, but be sure to check the edits! Some changes are being made... > >Also! Thank you so much for the silver et al, and for all the great advise! A lot of people are explaining that we had plastics in antiquity if you consider anything malleable ("plastic") or anything with polymers to be plastic. Thanks for the learning opportunity! But to be clear, I intended to mean oil-based plastic, like (in this case) polyethylene: what our plastic bags and ... 2 answersEnergy levels: 2, 8, 6 · Orbitals: 1s2 2s2 2p6 3s2 3p4 · If you need to fill in the little boxes, here's one for you. Each arrow represents one electron. You draw ... - In the electronic configuration of sulphur, the first two electrons will occupy 1s orbital. Since 1s orbital can hold only two electrons, the following ...1 answer · Top answer: Hint: Orbital diagrams are pictorial illustrations of the electrons present within an atom. The formation of orbital diagrams follow the AufBau Principle, ...
Hey Guys, ​ I was wondering if there are some databases for molexular orbital diagrams of more unusual compounds like phosphaalkenes or sulfur nitrides. I wanted to include some in a presentation ​ thanks for any help!
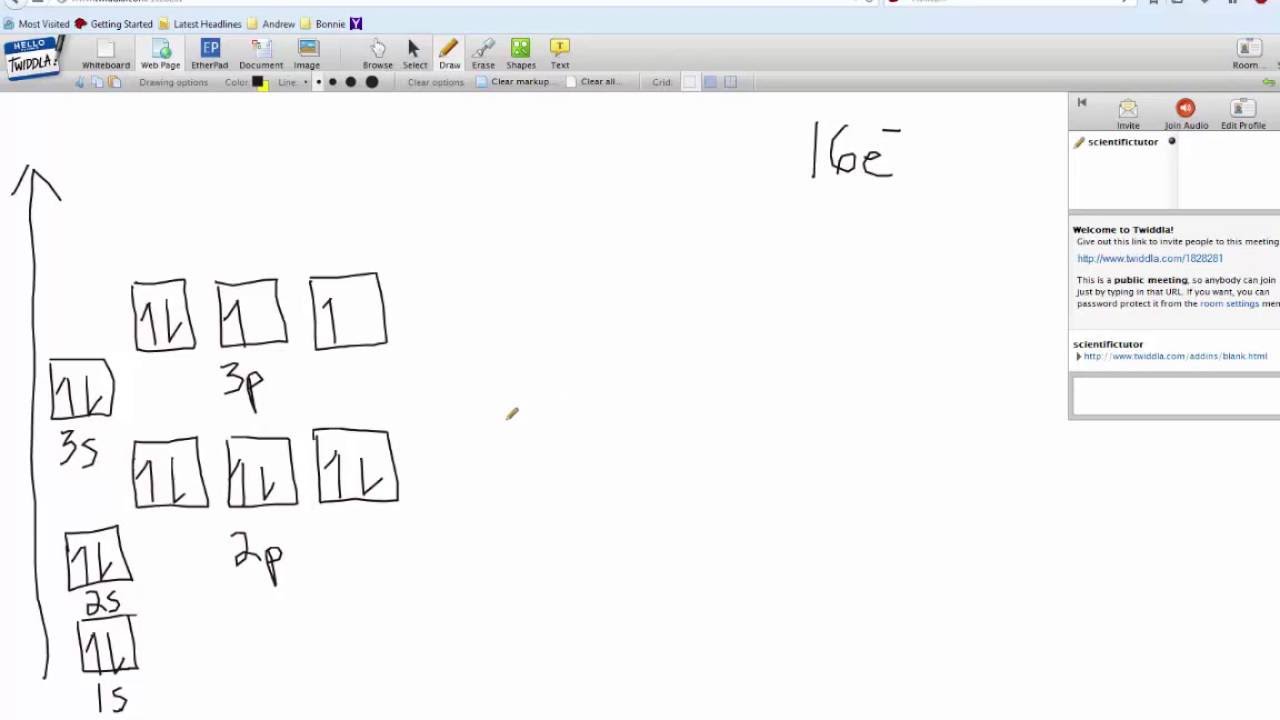
In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 ...Oct 24, 2016 · Uploaded by Wayne Breslyn
I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c...
*Originally posted in* [*/r/DestinyLore*](https://www.reddit.com/r/DestinyLore) # It's Hive, so I'm sure it's going to be strange. But at its core, their "magic" is still just science[.](https://bungie.net/common/destiny_content/grimoire/hr_images/603040_865612de633ac3e8a3c8c793f6fd0122.jpg) – [Ana Bray](https://www.ishtar-collective.net/transcripts/adventure-deathly-tremors) I’ve wanted to make this post for some time now and have been considering the science of Hive Magic since Stasis was r...
May 30, 2020 — The electron configuration for sulfur is 1s 2 2s 2 2p 6 3s2 3p4 and can be represented using the orbital diagram below. orbital diag sulfur.png ...
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
[Original post I made in /r/Astronomy](https://www.reddit.com/r/Astronomy/comments/3fda00/atmospheres_from_stars_to_planets_everything_you/) Hey, /r/space! I recently answered a [question](https://www.reddit.com/r/askscience/comments/3esx20/how_does_pluto_sustain_any_kind_of_atmosphere/) on /r/AskScience about how Pluto can sustain an atmosphere while the Moon does not. This got me thinking that perhaps people would like to know more about atmospheres as an astronomical phenomenon in genera...
Jan 26, 2021 — when we the electron configuration of Sulfur the first two electrons go in the 1s orbital. As 1s only hold two electrons and the next two ...
Sulfur (S) has an atomic mass of 16. Find out about its chemical and physical ... Electron Configuration, [Ne] 3s2 3p4. 1s2 2s2 2p6 3s2 3p4. Orbital Diagram.

















0 Response to "36 orbital diagram for sulfur"
Post a Comment