35 magnesium electron dot diagram
Magnesium Iodide (MgI2 or I2Mg) is an ionic compound.One magnesium atom loses two electrons, so it becomes +2 chargeTwo iodine atoms gain those two electrons... Step 4. Search for the bond forming between the magnesium and oxygen atoms: The ionic bond will be formed between the atoms as magnesium will be donating two of its valence electrons from the 3s shell to fulfill the electron deficiency in the oxygen atom. Step 5. Now draw the Lewis structure of magnesium oxide (MgO): From the diagram, it is ...
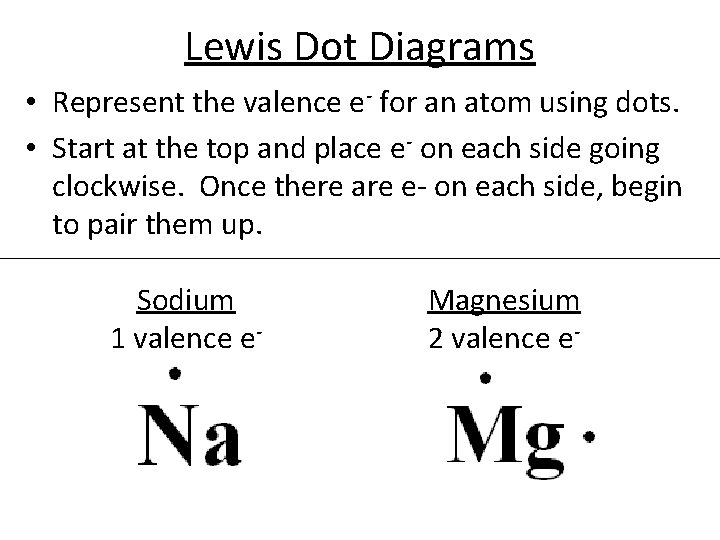
Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li Magnesium 12 24 12 12 12 Mg: Boron B5 11 5 6 5 Element Atomic # Atomic Mass ...

Magnesium electron dot diagram
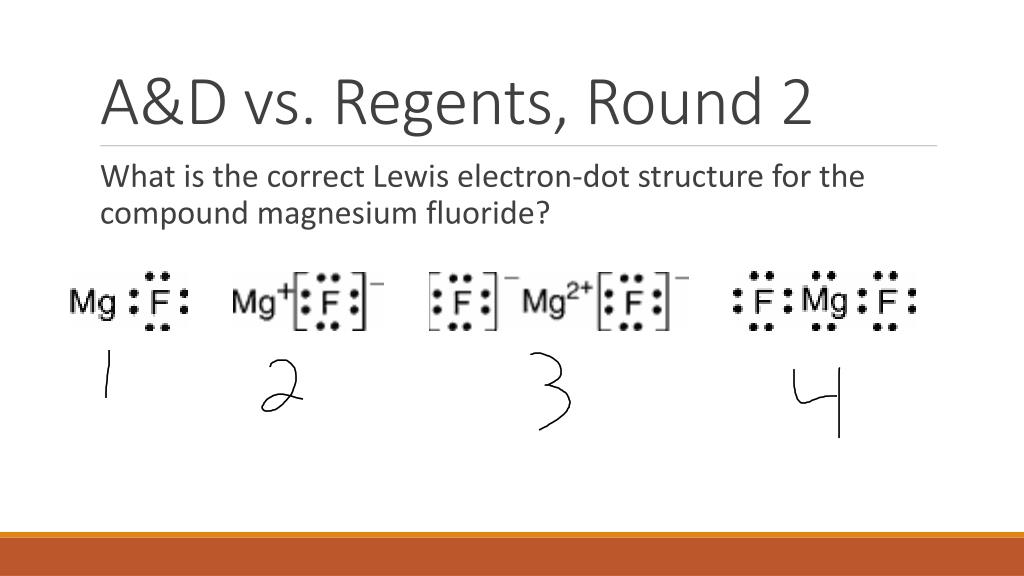
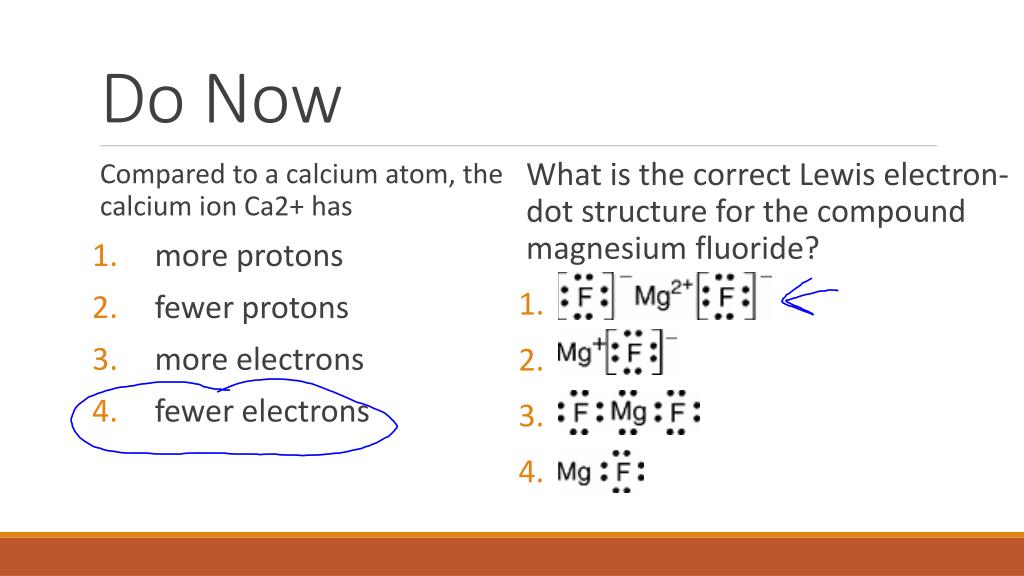
Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?. MgF2 (2 should be subscript) because fluoride is -1 electron and Mg is 2+ d) draw the dot diagram for magnesium fluoride. since this is an. Answer (1 of 3): draw a magnesium symbol and dot two dots around the symbol. Hopes this helps:) 18.02.2020 · Although magnesium-based cofactors are highly active in biochemical reactions, magnesium-based materials generally exhibit poor catalytic activity for oxygen reduction. Here the authors enhance ...
Magnesium electron dot diagram. Hence, the electron dot diagram for magnesium oxide consists of magnesium in M g 2 + form, and oxygen shown with 8 valence electrons (dots) with a negative charge of 2. Note: The electron dot diagram of any atom or molecule is also called the Lewis dot structure of atoms. This representation consists of rules like, total valence electrons are ... [DIAGRAM] Lewis Electron Dot Diagrams Magnesium . File:Lewis dot Mg svg Wikimedia Commons . Lewis Dot Diagram For Phosphorus Wiring Diagram Source . Magnesium Fluoride Facts, Formula, Properties, Uses . P2 Lewis Structure / Chapter 5 Chemical Bonds P2 / Write ... Describe the electron dot diagram system of representing structure. Draw electron dot diagrams for elements. How do we show electrons in atoms? Diagrams contain a lot of useful information in a compact format. What does the diagram above tell us? The football play diagrammed above describes the lineup of each player on the team and describes how they will move when the ball is snapped ... Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, …
1. Draw an "electron dot" diagram showing the first 18 elements in the periodic table. 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of ions and ionic compounds. 4. Explain how hydrogen can be considered as behaving like a metal or a nonmetal. 03.07.2019 · Lewis Electron Dot Diagrams . Lewis electron dot diagrams may be drawn to help account for the electrons participating in a chemical bond between elements. A Lewis diagram counts the valence electrons. Electrons shared in a covalent bond are counted twice. For the octet rule, there should be eight electrons accounted for around each atom. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons. Element: Bohr Diagram; Group Number (PT) # of Valance Electrons; Lewis Dot Structure; Calcium. Carbon. Hydrogen. Helium. Oxygen. Fluorine. Neon. Sodium. Aluminum. Determining the Ionic Charge. Element ... Click here👆to get an answer to your question ️ Draw an electron dot diagram to show the formation of each of the following compounds:Magnesium Chloride. [H = 1, C = 6, Mg = 12, Cl = 17] .
Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is ... "Cl" can get a noble gas s^2p^6 configuration by gaining an electron and forming a chloride ion, "Cl"^"-". The Lewis structure of "MgCl"_2 is therefore (From www.superteachertools.net) The compound is held together by electrostatic attractions between the positively charged magnesium ion and the negatively charged chloride ions. The electron from a sodium atom transfers to a chlorine atom. Modelling ionic bonding. The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 2p^5#
The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. What is an bond electron transfer how electron dot diagram for s awesome sulfur atom flow block orbital diagram for magnesium awesome lewis electron dot diagrams. Magnesium reacts with sulfur to produce sulfide a in.
It is so because the Lewis structure is drawn for such molecules where sharing of valence electrons takes place, which is the case with magnesium fluoride. Also called electron dot structures, the Lewis diagrams help with studying the reasons behind the atoms within a molecule achieving the most stable position to remain unexcited.
Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?.Dec 18, · Best Answer: Magnesium has 2 valence electrons and Fluorine has 7 valence electrons in order for the 2 elements to combine, you need 1 Mg atom and 2 F atoms The Mg gives one ...
: F : Mg : F : .. .. Magnesium has two electrons on its outer shell Each of the electrons will be shared with a Florine atom. This results in a compound MgF_2 Each Florine starts with seven electrons around the atom, combining with the Magnesium atom give the Florine eight electrons around each Florine atom. This eight electrons are found in four pairs. shown in the diagram as a pair on top a ...
electron dot diagram for Magnesium. electron dot diagram for Iodine. electron dot diagram for Boron. electron dot diagram for Sulfur. electron dot diagram for Carbon. ... Lewis structure for ONCl. 4 dots around N, double bonded to O, 2 dots around O, single bonded to Cl, 6 dots around Cl.
Does the model correctly represent the electron dot diagram of magnesium? Why or why not? Select two options. M g with 1 dot above and 1 right. Yes, because the chemical symbol of magnesium is Mg. No, because the chemical symbol of magnesium is Mn. Yes, because magnesium has two valence electrons. No, because magnesium has seven valence electrons.
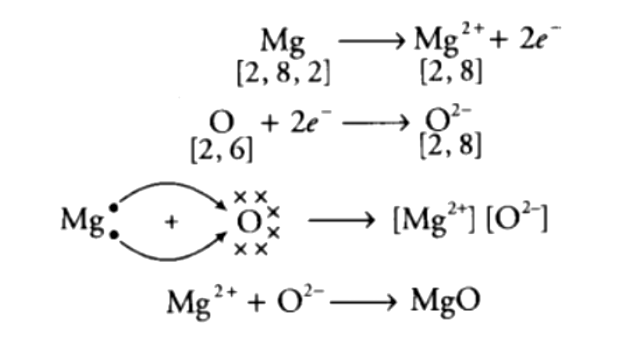
asked Aug 13, 2019 in Class X Science by muskan15 Expert (37.9k points) (i) Write electron-dot structures for magnesium and oxygen. (ii) Show the formation of MgO by the transfer of electrons. (iii) What are the ions present in this compound? metals and non-metals.
Since the lewis electron dot diagrams are based on the number of valence electrons it would hold true that the elements in the same group would have the same electron dot diagram. The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons.
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element.
Magnesium ribbon burns in air with an extremely bright white light, giving off a large amount of energy, and white smoke with is mostly magnesium oxide in very fine particles. The magnesium ribbon easily crumbles into a white powder which is a mixture of magnesium oxide (about 90%) and magnesium nitride (about 10%).
What is the electron dot diagram for magnesium nitride? Magnesium Nitride is Mg3N2. What I think you do is draw it Mg N Mg N Mg and then draw 8 electrons around each Nitrogen so that Mg shares its ...
Nov 15, 2017 - electron diagram of magnesium electron dot structure for - 28 images - understanding the lewis dot structure with exles, dot cross diagram magnesium fluoride, calcium ground state electron configuration the, 1 write the electron dot structure for sodium oxygen, draw the electron dot structure for formation of magnesium
12.12.2017 · magnesium chlorine e) In the box below, draw the Lewis electron-dot structure for the compound formed from magnesium and chlorine. [ Include any charges or partial charges.] (1 pt.) 32) Explain, in terms of electronegativity, why an H-F bond is expected to be more polar than an H-I …
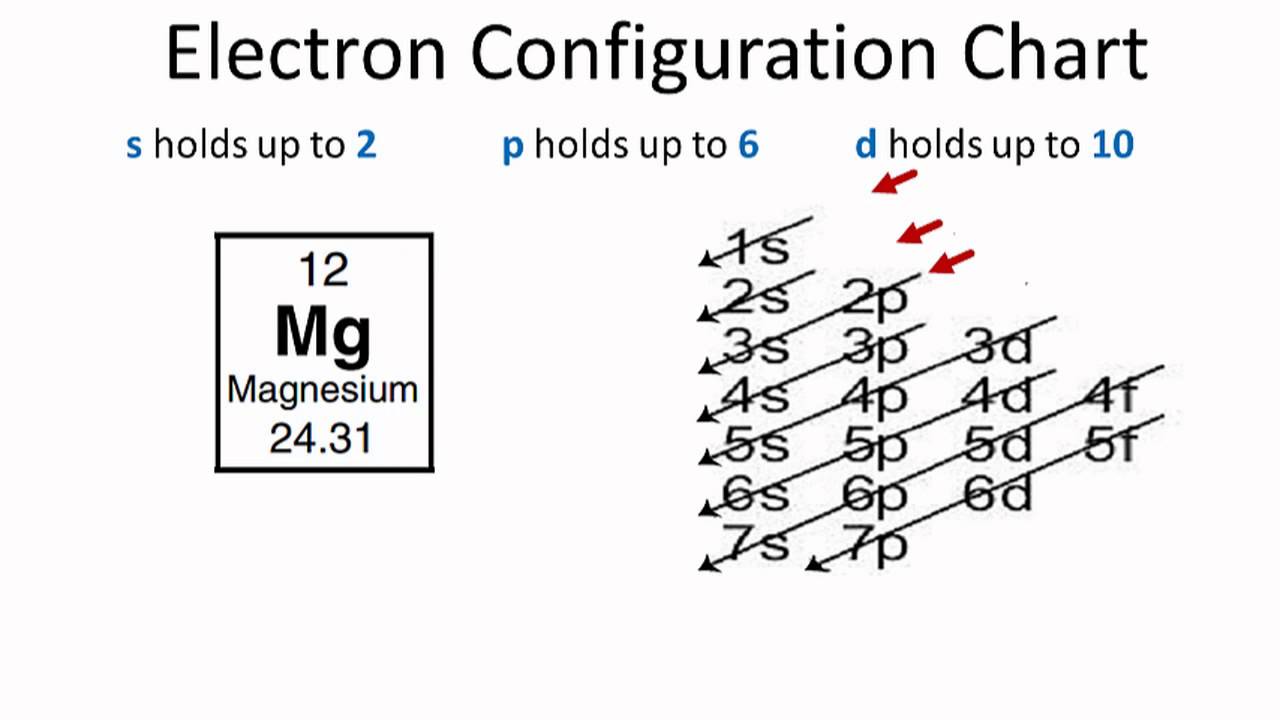
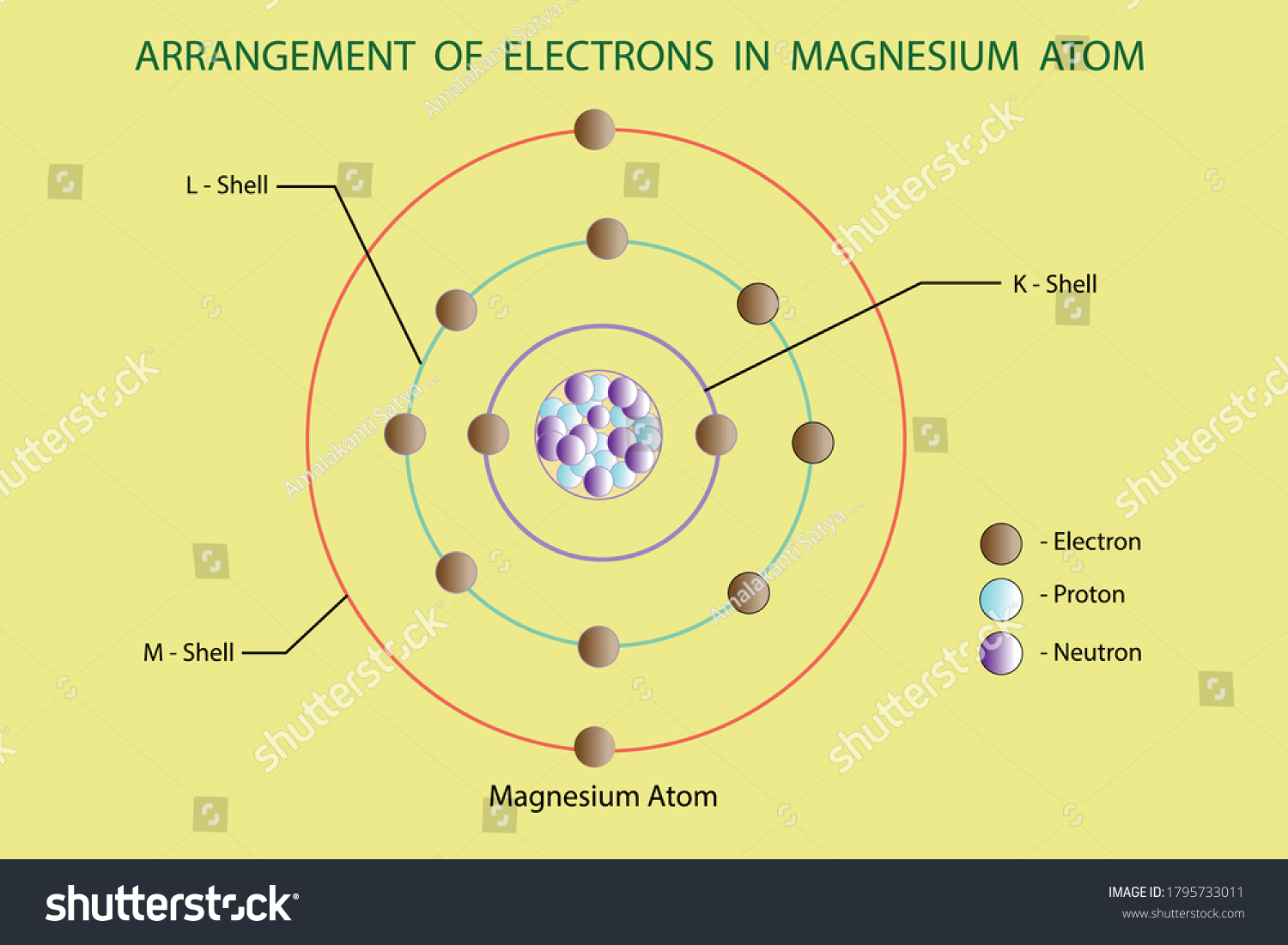
Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. Video: Magnesium Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to ...
Magnesium phosphide (Mg3P2 or P2Mg3) is IONIC because it is a combination of a metal and non-metal.Each magnesium, of which there are three, LOSES two electr...
Find Similar Structures. Molecular Formula. Mg3(PO4)2 or Mg3O8P2. Synonyms. magnesium phosphate. TRIMAGNESIUM PHOSPHATE. 7757-87-1. Phosphoric acid, magnesium salt (2:3) Magnesium phosphate anhydrous.
what is the electron dot diagram for magnesium oxide well magnesium oxide is an ionic species which we could represent as mg 2 o 2 elemental magnesium has 12 nuclear protons z=12 it has 2 valence electrons that are conceived to be lost when it undergoes oxidation to mg 2 mgrarrmg 2 2e i elemental atomic oxygen has 8 electrons z=8. Download.
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core.
The electron dot representation of Magnesium oxide is given above. Was this answer helpful? 0. 0. Similar questions. In N H 4 C l O 4 , what is the charge on N H 4 in lewis structure of molecule? Medium. View solution >
Electron dot diagram of a Magnesium atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Magnesium, we got to know, it has only 2 valence electrons. So, just represent the 2 valence electrons around the Magnesium atom as a dot.
18.02.2020 · Although magnesium-based cofactors are highly active in biochemical reactions, magnesium-based materials generally exhibit poor catalytic activity for oxygen reduction. Here the authors enhance ...
Answer (1 of 3): draw a magnesium symbol and dot two dots around the symbol. Hopes this helps:)
Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?. MgF2 (2 should be subscript) because fluoride is -1 electron and Mg is 2+ d) draw the dot diagram for magnesium fluoride. since this is an.

















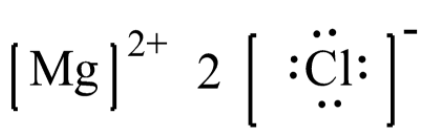

0 Response to "35 magnesium electron dot diagram"
Post a Comment