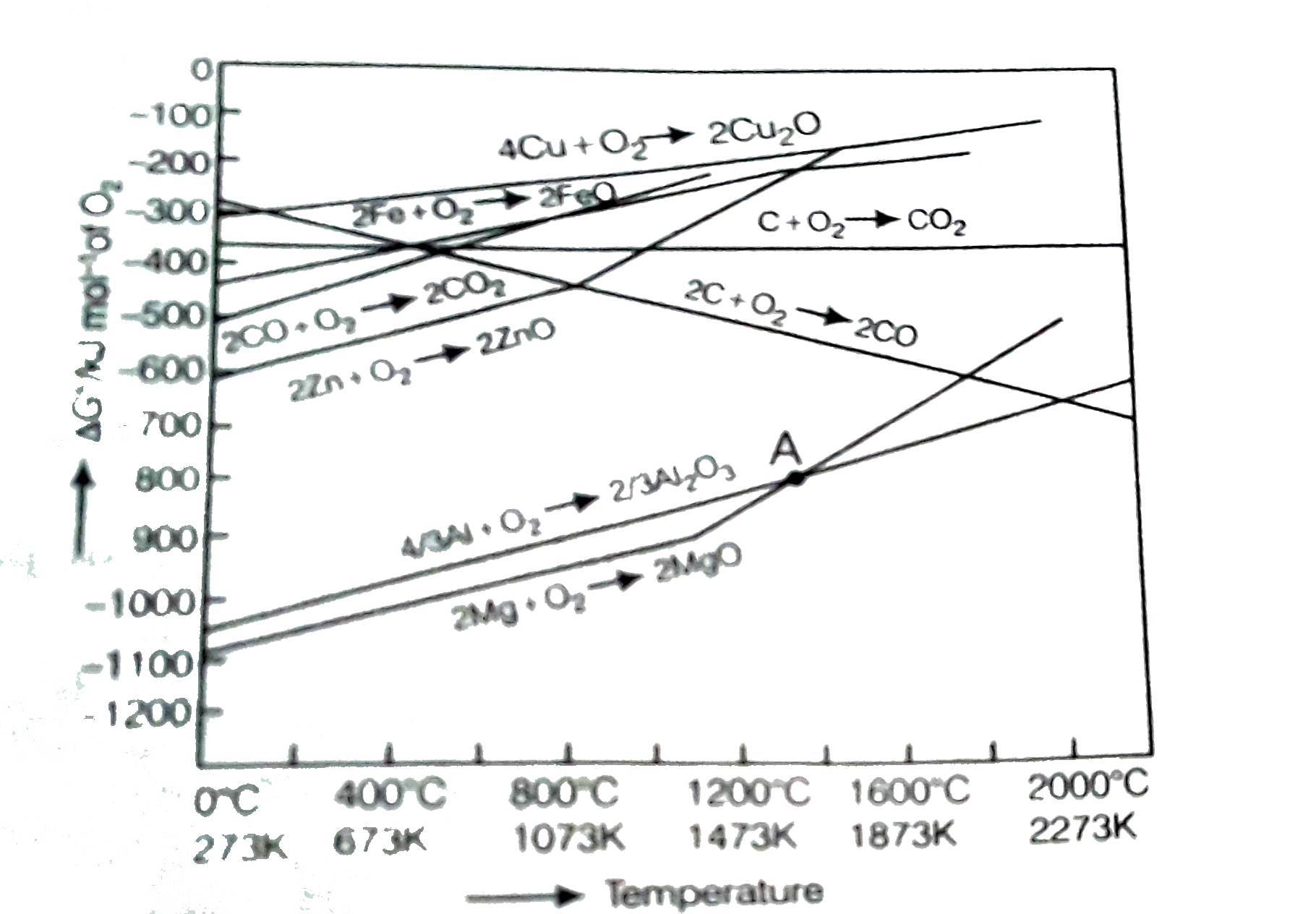
34 ellingham diagram for oxides
Ellingham Diagram. Growth rates of selected oxides CoO>NiO>Cr2O3>Al2O3. Thermodynamic stabilities of selected oxides Al2O3>Cr2O3>CoO>NiO. Effect of Electrical Current on Oxidation Rate. x. Cations Electrons Anions Cation Vacancies Metal Oxide Gas MO M 2e1 2. O. 2MO. 2++−+ = +−=2− 2 2 12e O or M+O2−=MO+−.
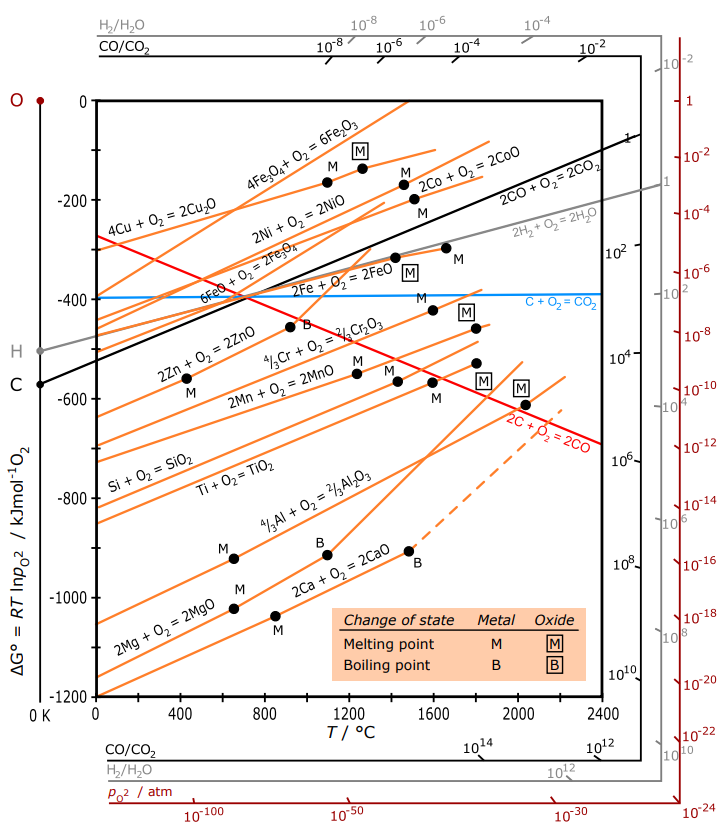
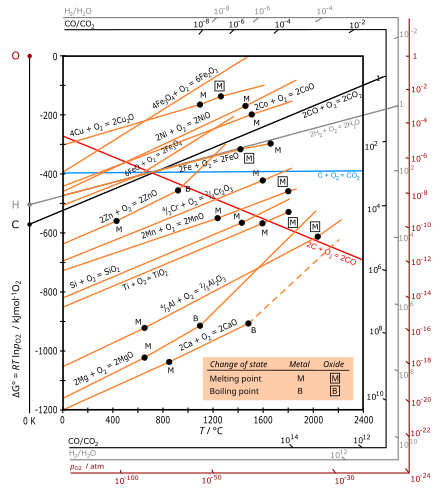
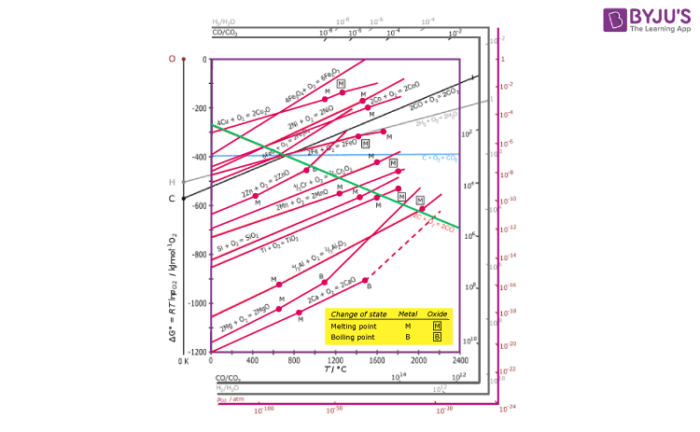
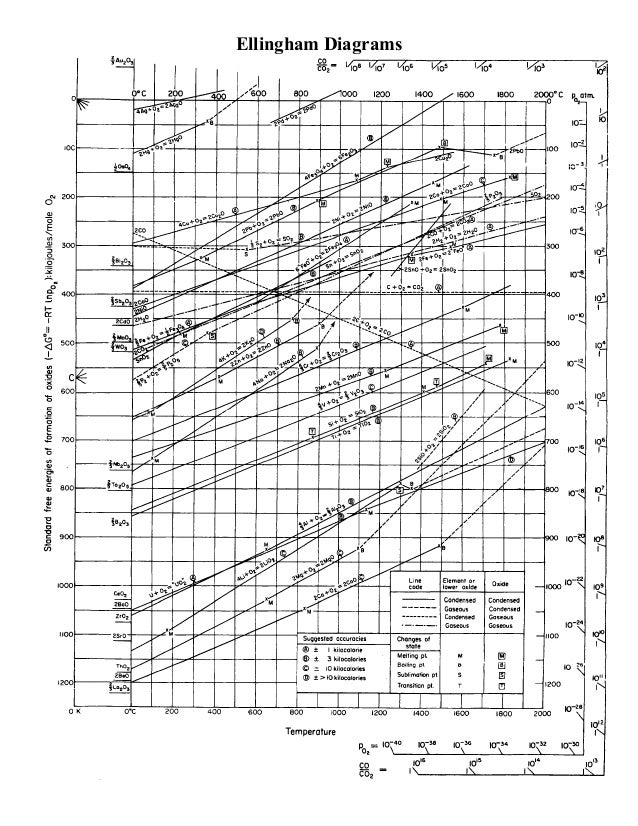
Ellingham diagram theory pO 2 scale For example, the Ellingham diagram for oxides includes species for which the formation reaction can be presented as: Me A O B B Me O g B A 1 ( ) 2 1 2 (4) In this reaction, the oxygen element balance is equal to 1 (the default option in HSC diagrams), which gives the 1/2 coefficient for O 2(g).
Salient Features. Curves in the Ellingham diagrams for the formation of metallic oxides are straight lines with a positive slope. The lower the position of a metal in the Ellingham diagram, the greater is the stability of its oxide. For example, the Ellingham diagram for Al is found to be below Fe 2 O 3. The greater the gap between any two ...

Ellingham diagram for oxides
Reducing agents A major application of the Ellingham diagram is the determination of the conditions required to reduce metal compounds, such as oxides or sulphides, to obtain pure metal. This is often the basis of extraction metallurgy, the extraction of metals from their ores. It is also very important in the recycling of metals.
Download scientific diagram | Ellingham diagram for different oxides [9-11] from publication: Reduction of Oxide Minerals by Hydrogen Plasma: An Overview | Carbothermic reduction of oxide ...
2. Ellingham diagram is used to predict thermodynamic feasibility of reduction of oxides of one metal by another metal. Any metal can reduce the oxides of other metals that are located above it in the diagram. For example, in the Ellingham diagram, for the formation of chromium oxide lies above that of the aluminium, meaning that Al 2 O 3
Ellingham diagram for oxides.
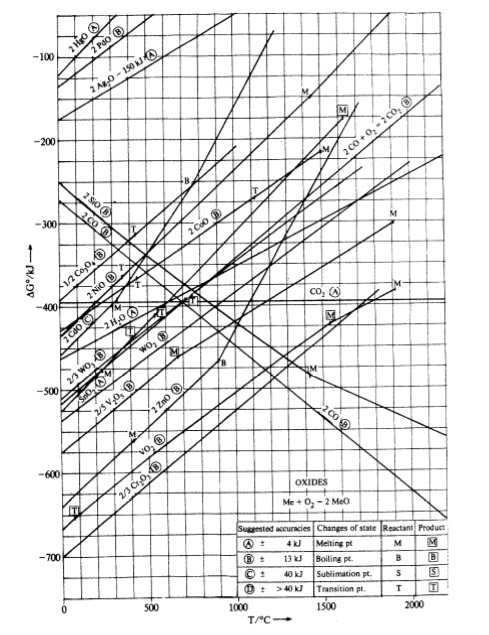
Ellingham Diagrams Stanley M. Howard, SD School of Mines and Technology Standard Gibb's Energies of Formation for Bromides Chlorides Fluorides Hydrides Iodides Nitrides Oxides Sulfides Selenides Tellurides The figures include nomographs for equilibrium partial pressures. Data sources: 1) Thomas B. Reed, Free Energy of Formation of Binary Compounds,
Download scientific diagram | The Ellingham diagram of Nd, Fe and B oxides from publication: Recycling the Rare Earth Elements from Waste NiMH Batteries and Magnet Scraps by Pyrometallurgical ...
Rare Earth Oxides Ellingham Diagram.xls Author: showard Created Date: 1/23/2014 4:29:04 PM ...
Harold Johann Thomas Ellingham (1897-1975) was a British physical chemist and is best known for the diagrams named after him that plot the change in standard free energy with respect to temperature for reactions like the formation of oxides, sulfides and chlorine of various elements, such as: 2x/ y M + O 2 ↔ 2 x/ y M x O y
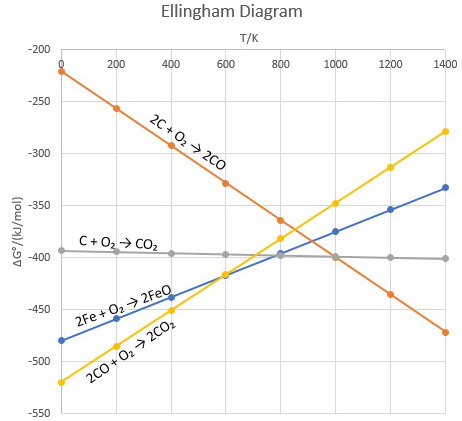
An Ellingham diagram normally consists of plots of change in the Gibbs energy with temperature for the formation of oxides. An Ellingham diagram for oxides has several important features. (i) The graphs for most metal to metal oxide reactions show a positive slope. Ex: 2M + O2 →2MO.
General Conclusions of Ellingham Diagram : The main characteristic features of the diagram are given below: → The curve for the formation of metal oxide from its metal by the reaction of oxygen always slope upwards because the value of ∆G° increases with increase in temperature. → Some curves show a sudden change in the slope.
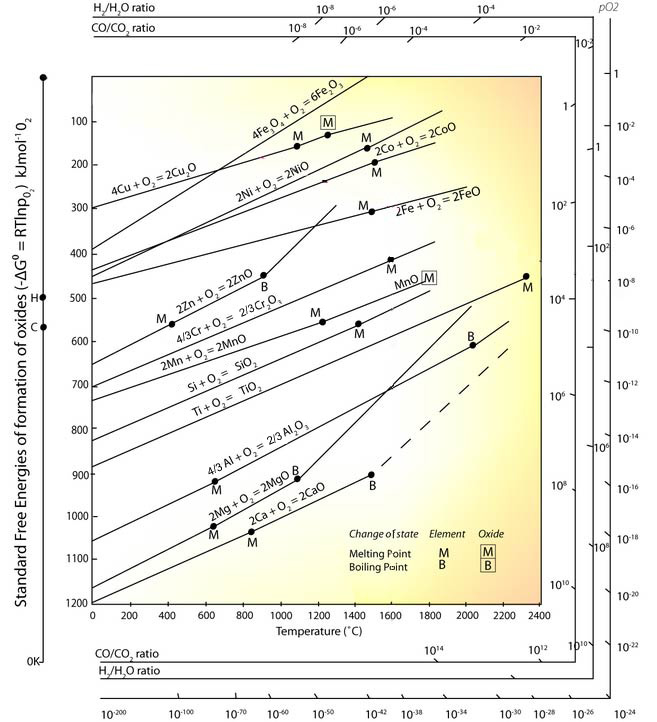
Ellingham diagrams were 1stconstructed by Harold Ellingham in 1944. (image will be updated soon) Following are the Salient Features of it – 1. It is a plot of ∆G°in kJ/mol of oxygen and temperature of formation of oxide. 2. As ∆G°becomes less negative at high temperatures so each line of converting metals into metal oxide slope upwards. 3. Each plot line is straight line except some lines where the change in phase such as solid 🡪 liquid or Liquid 🡪 Gas etc. takes place. 4. With increase in temperature slope lines cross ∆G°= 0 which means for them ∆G°>0. Theoretically this happens in case of mercury, silver and gold.
The Ellingham Diagram, originally constructed for oxides, is a tool to find a variety of thermodynamic data quickly, without the need for repetitive calculation. The diagram is essentially a graph representing the thermodynamic driving force for a particular reaction to occur, across a range of temperatures.
The Ellingham diagram shown is for metals reacting to form oxides (similar diagrams can also be drawn for metals reacting with sulfur, chlorine, etc., but the oxide form of the diagram is most common). The oxygen partial pressure is taken as 1 atmosphere, and all of the reactions are
In metallurgy, the Ellingham diagram is used to predict the equilibrium temperature between a metal, its oxide, and oxygen — and by extension, reactions of a metal with sulfur, nitrogen, and other non-metals. The diagrams are useful in predicting the conditions under which an ore will be reduced to its metal.
the ellingham diagram ellingham1 plotted experimentally determined standard free energy of formation (∆g0) of various oxides (and sulfides) using one mole of oxygen with temperature ellingham found that, the standard enthalpy and entropy of formation of a compound don't change significantly with temperature as long as there is no change …
Ellingham diagram for Reduction of Oxides: Ellingham diagram is a plot between Δ f G o and T for the formation of oxides of metals. A general reaction expressing oxidation is given by: 2xM(s) + O 2 (g) → 2M x O(s) As is evident from the reaction, the gaseous amount of reactant is decreasing from left to right as the product formed is solid metal oxide on the right side.
The study of Ellingham diagrams for common metallurgical processes shows that. 1. At temperature above 1073 k, coke will be reducing the FeO, and itself be oxidised to CO. 2. Reduction of F e 2 O 3 and F e 3 O 4 can be carried out by relatively lower temperature ( 500 - 800 k) by CO. 3.
I. PRINCIPLES OF EXTRACTIVE METALLURGY RAKES H KUMAR Email : rakesh@ninlindia.org Extractive metallurgy as a discipline deals with the extraction of metals from naturally occurring
Ellingham Diagram. An Ellingham diagram shows the relation between temperature and the stability of a compound. It is basically a graphical representation of Gibbs Energy Flow. In metallurgy, we make use of the Ellingham diagram to plot the reduction process equations. This helps us to find the most suitable reducing agent when we reduce oxides ...
An Ellingham diagram is a graph showing the temperature dependence of the stability of compounds. This analysis is usually used to evaluate the ease of reduction of metal oxides and sulfides.These diagrams were first constructed by Harold Ellingham in 1944. In metallurgy, the Ellingham diagram is used to predict the equilibrium temperature between a metal, its oxide, and oxygen — and by ...
The position of the line for a given reaction on the Ellingham diagram shows the stability of the oxide as a function of temperature. Reactions closer to the top of the diagram are the most “noble” metals (for example, gold and platinum), and their oxides are unsta ble and easily reduced. As we move down toward the bottom of the diagram, the metals become progressively more reactive and their oxides become harder to reduce.
Nov 20, 2021 · The Ellingham Diagram is a graph of Gibbs energy (ΔG) versus Temperature (T) for the formation of oxide of a metal. The diagram represents how the stability of compounds is dependent on the temperature. It is widely used in the identification of the most suitable reducing agent for the reduction of metal ores.
Ellingham diagram is used to predict thermodynamic feasibility of reduction of oxides of one metal by another metal. Any metal can reduce the oxides of other metals that are located above it in the diagram.
The Ellingham diagram shown is for metals reacting to form oxides (similar diagrams can also be drawn for metals reacting with sulfur, chlorine, etc., but the oxide form of the diagram is most common). The oxygen partial pressure is taken as 1 atmosphere, and all of the reactions are normalized to consume one mole of O2. ...
English: The Ellingham diagram is giving the free energy of formation of metal oxides and the corresponding oxygen partial pressure at equilibrium. Labels in English. Date: 30 March 2016: Source {{Self-made with most data from here }} Author: DerSilberspiegel: Licensing .
The Ellingham diagram for the oxides is a useful thermodynamic tool for determining chemical reactions and equilibria associated with various materials processes such as heat treating, brazing, decarburizing, case hardening, etc.
The Ellingham diagram suggests that all the oxides of iron and phosphorus would get reduced during reduction smelting of MnO, Cr2 O 3, as well as SiO 2. As a result, the overall grade of the molten alloy is determined by the extent of oxide reduction and the burden of iron oxides and other easy-to-reduce oxides in the charge.



![Schematic of the Ellingham diagram for selected oxides [33,34]. Mg ...](https://www.researchgate.net/profile/Jayesh-Patel-19/publication/303695454/figure/fig2/AS:459458577997826@1486554869464/Schematic-of-the-Ellingham-diagram-for-selected-oxides-33-34-Mg-oxidation-is-marked.png)

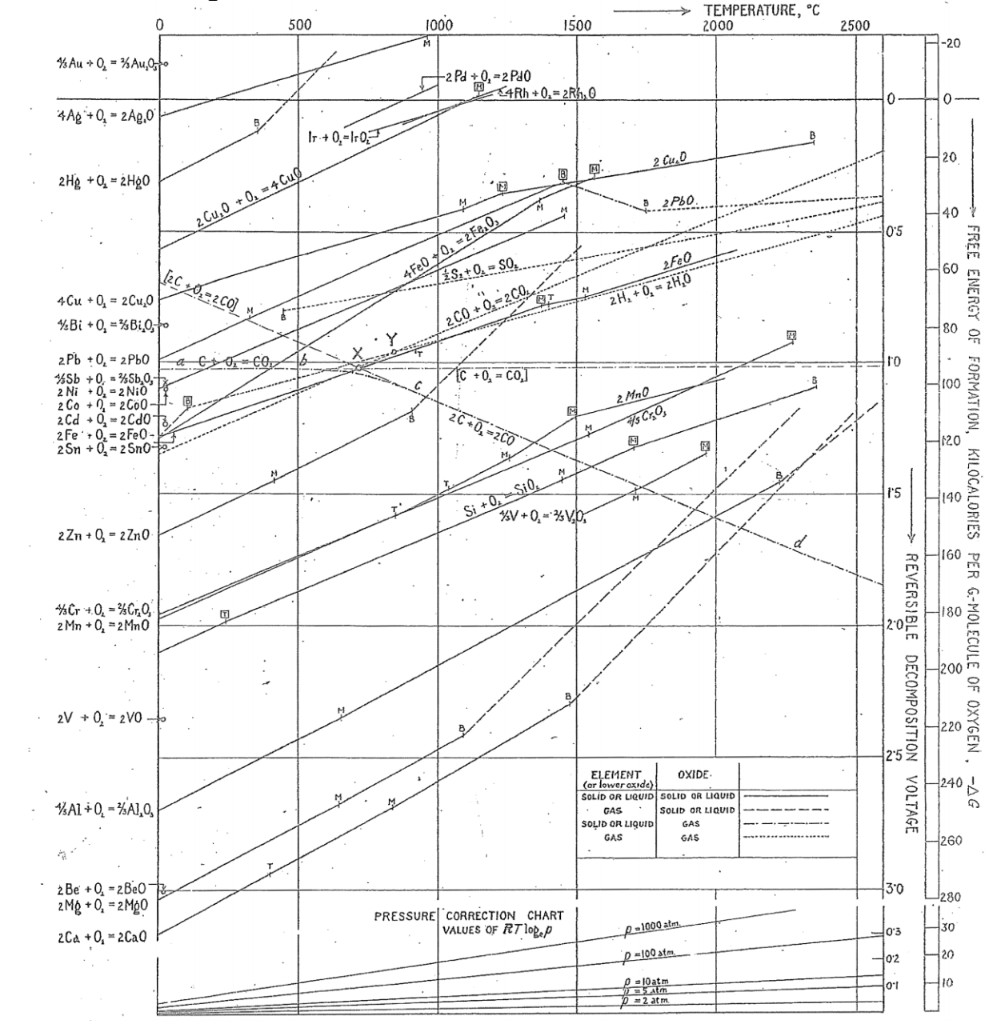


![Ellingham diagram for different oxides [9–11] | Download ...](https://www.researchgate.net/profile/Kali-Sabat/publication/257139583/figure/fig1/AS:392582355013637@1470610335446/Ellingham-diagram-for-different-oxides-9-11.png)





.bmp)









0 Response to "34 ellingham diagram for oxides"
Post a Comment