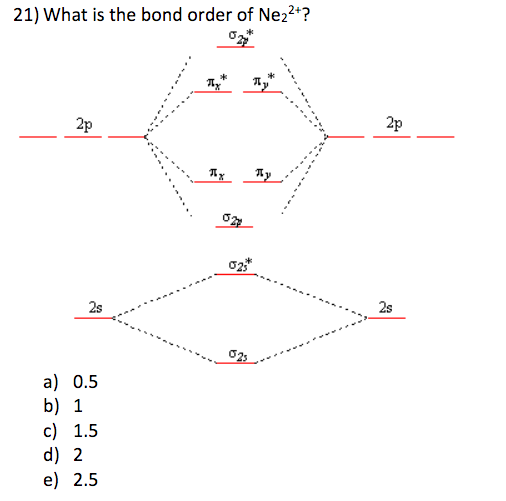
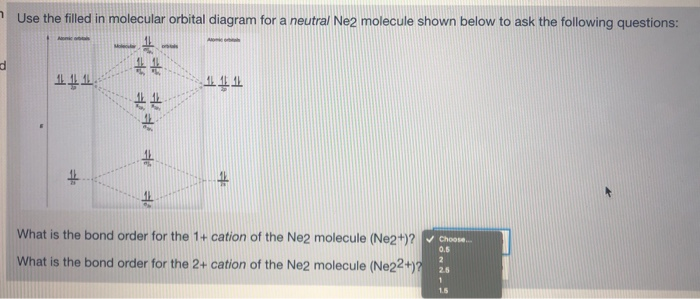
39 molecular orbital diagram for ne2 2+
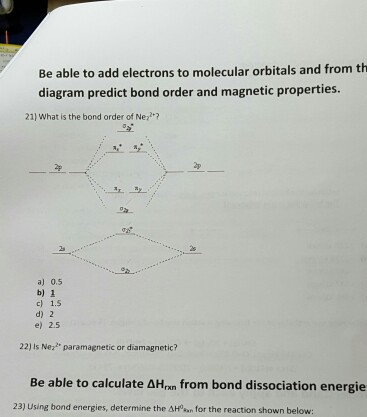
To form the 2+ ion, the uppermost electrons in the sigma* 2p orbital are removed, making it isoelectronic with F2, so it has a bond order of 1 and should be ...1 answer · 0 votes: Question: Is Ne2 2+ paramagnetic or diamagnetic? To understand this answer you have to know ... For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. Give each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order. Rationalize the trend in bond order in terms of bond strength.
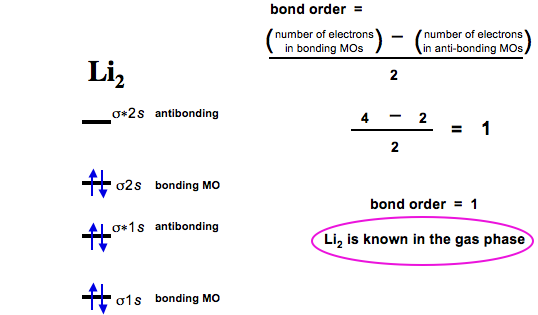
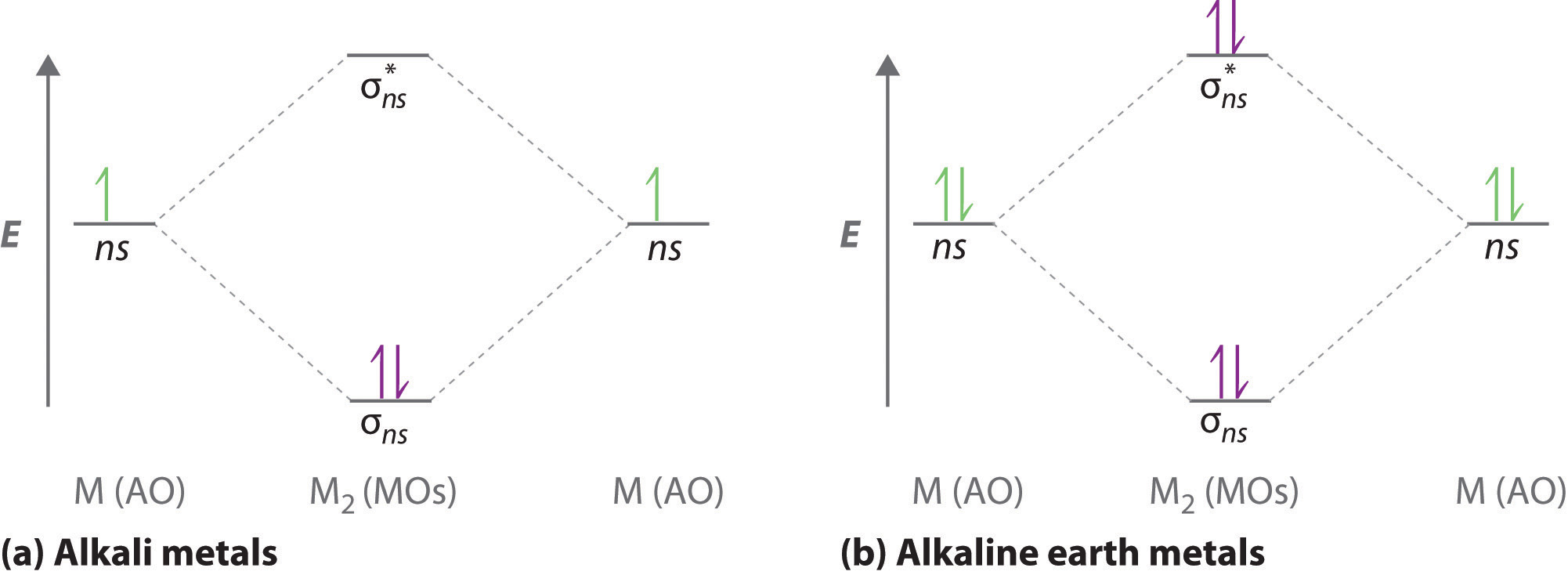
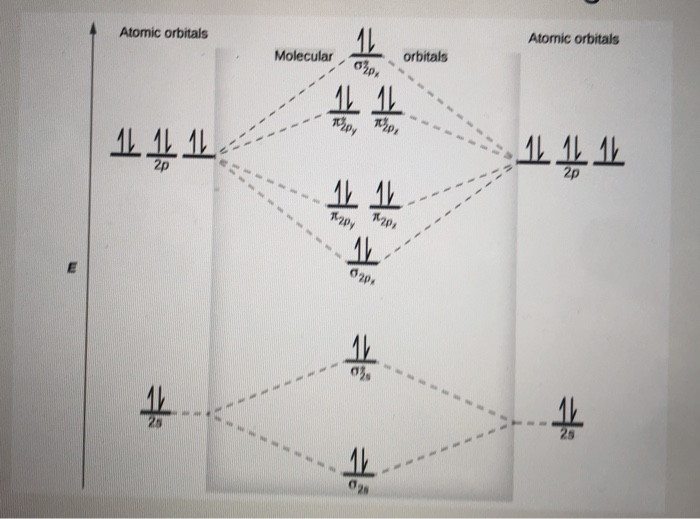
There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...

Molecular orbital diagram for ne2 2+
2:36Molecular Orbital Diagram for Nitrogen Gas (+1 ion) (N2(+)). ... and it is Paramagnetic. sigma2s(2),sigma2s*(2 ...9 Jun 2017 · Uploaded by chemistNATE Draw the molecular orbital diagram for Ne2+ and determine if the bond ... In Ne2 ion, the electronic configuration of Ne is Ne 1s2s22p6 Calculate the total ... Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below.
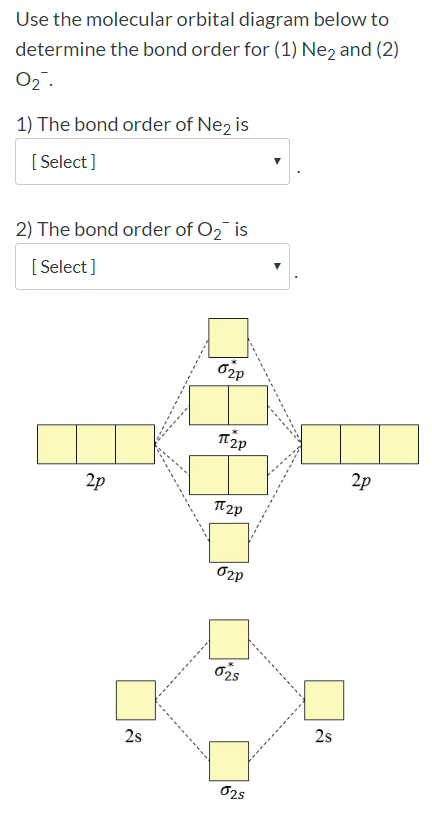
Molecular orbital diagram for ne2 2+. 0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d... Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable. what is the bond order of Ne2 2+. i know its 1 but I do not know how to use this diagram. can someone explain this? Show transcribed image text. Expert Answer. In this case, the difference is the H-X-H bond angle which decreases from o to 90 o Molecular Orbital Theory – . Item 2: Part A Complete the MO energy diagram for the N2+ ion by dragging the electrons Electron with spin up., ↑, ↑↓, ↓ in the figure given below.M.O. diagram for N2+Molecular orbital diagram - Wikipedia
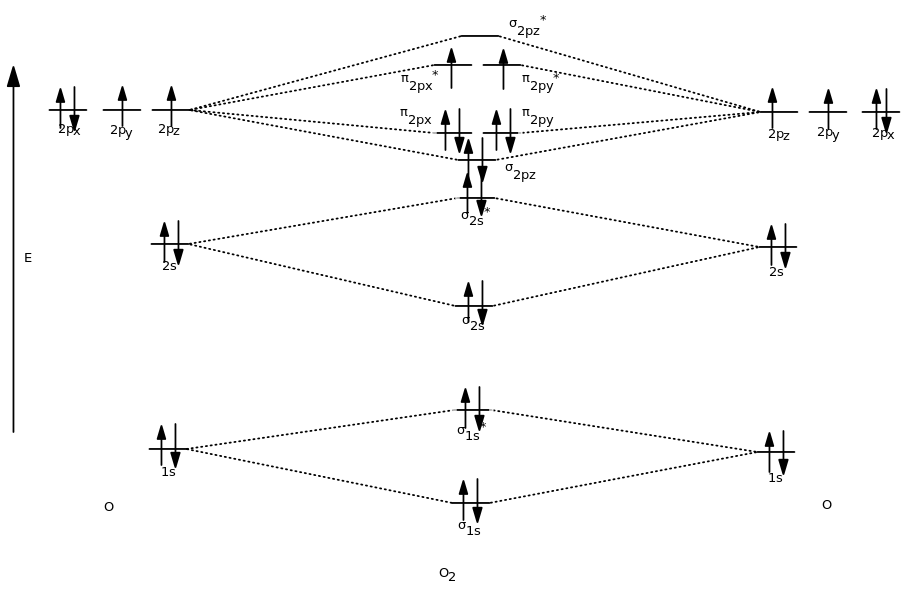
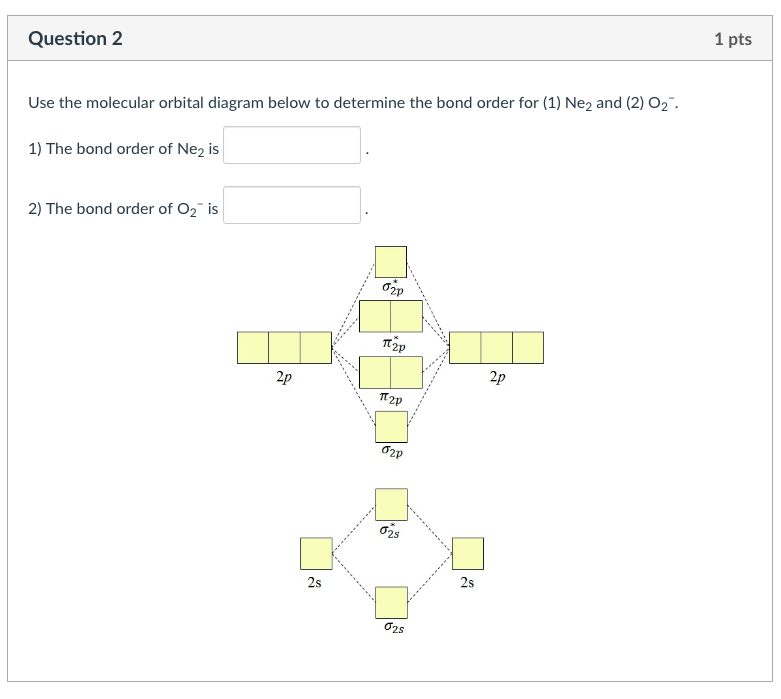
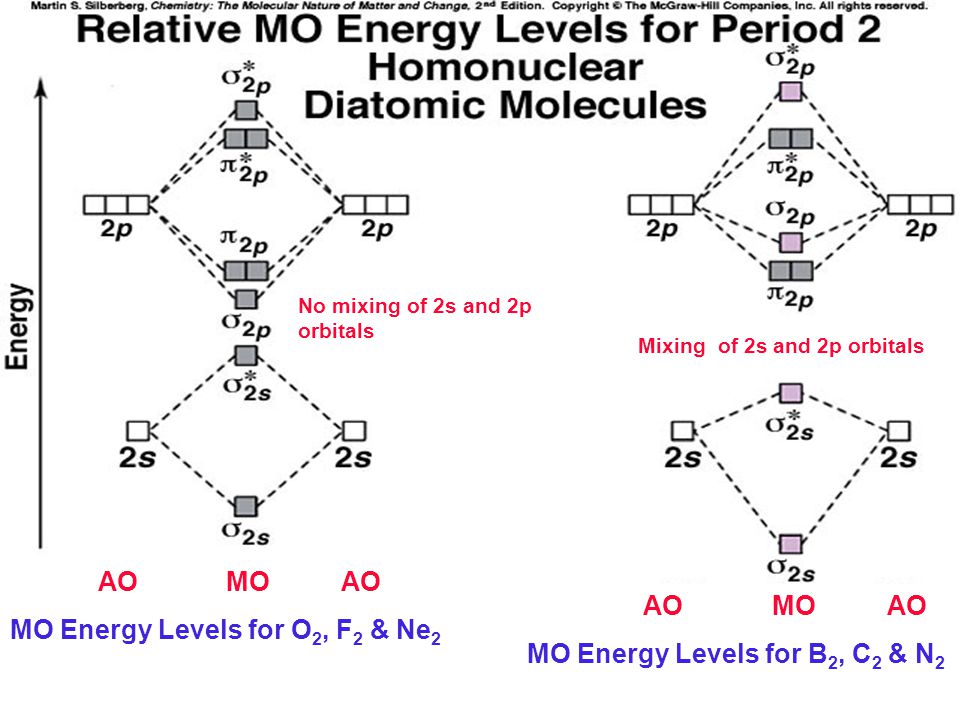
Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Page 1. MO Diagrams for Elements Li2 through Ne2. (Don't memorize.) Li2 through N2. O2 through Ne2. 4 Mar 2018 — Explanation: Neon atom has 10 electrons and its electronic configuration is . When molecule is considered, it has two neon atoms and thus is ...2 answers · 18 votes: Bond order of Ne2 = (10-10) =0 So Ne2 is unstable ;Ne2 cannot exist Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ... And the molecular orbital diagram is ne2 molecular orbital diagram luxury energy level diagrams hydrogen hypothetical 3 idealized mo diagram bonding in o2 f2 and ne2. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence ...
the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ... 13:21Molecular Orbital Diagram of Neon Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject ...27 Sep 2018 · Uploaded by Ekeeda Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below. Draw the molecular orbital diagram for Ne2+ and determine if the bond ... In Ne2 ion, the electronic configuration of Ne is Ne 1s2s22p6 Calculate the total ...
2:36Molecular Orbital Diagram for Nitrogen Gas (+1 ion) (N2(+)). ... and it is Paramagnetic. sigma2s(2),sigma2s*(2 ...9 Jun 2017 · Uploaded by chemistNATE
The Energy Of S2pz Molecular Orbital Is Greater Than P2px And P2py Molecular Orbitals In Nitrogen Molecule Sarthaks Econnect Largest Online Education Community
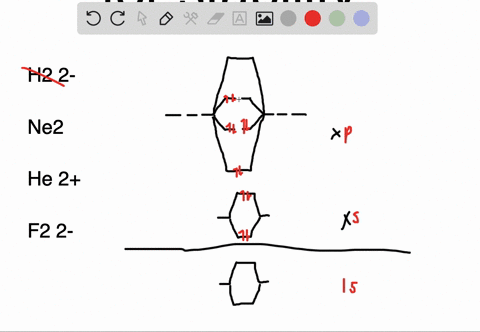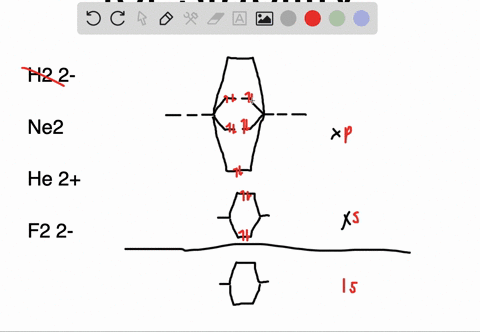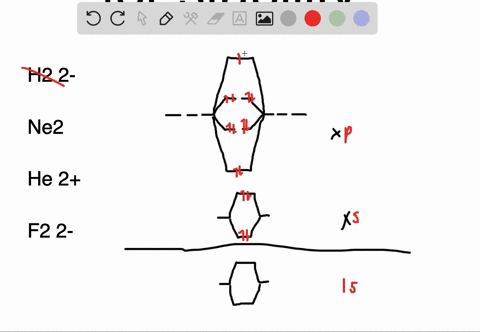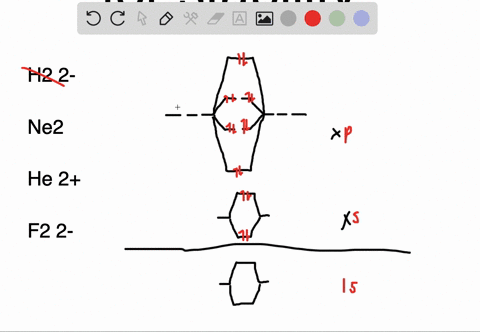
Solved Apply Molecular Orbital Theory To Predict If Each Molecule Or Ion Exists In A Relatively Stable Form A H2 2 B Ne2 C He2 2 D F2 2
Solved Apply Molecular Orbital Theory To Predict If Each Molecule Or Ion Exists In A Relatively Stable Form A H2 2 B Ne2 C He2 2 D F2 2

Draw The Molecular Orbital Diagram For Ne2 And Determine If The Bond Between The Two Atoms Homeworklib
















0 Response to "39 molecular orbital diagram for ne2 2+"
Post a Comment