39 democritus atomic model diagram
Here you will find curriculum-based, online educational resources for Chemistry for all grades. Subscribe and get access to thousands of top quality... Democritus' atoms were small, hard particles that were all made of the same material but were different shapes and sizes. Orbits are located at certain distances from the nucleus. 11 The Quantum (Wave) Model Today's atomic model Electrons do not move about an atom in a definite path...
The classic model of an atom was given by Ernest Rutherford called the Rutherford atomic model or Rutherford model of the atom. The concept of atom dates back to 400 BCE when Greek philosopher Democritus first conceived the idea. However, it was not until 1803 John Dalton proposed again the...

Democritus atomic model diagram
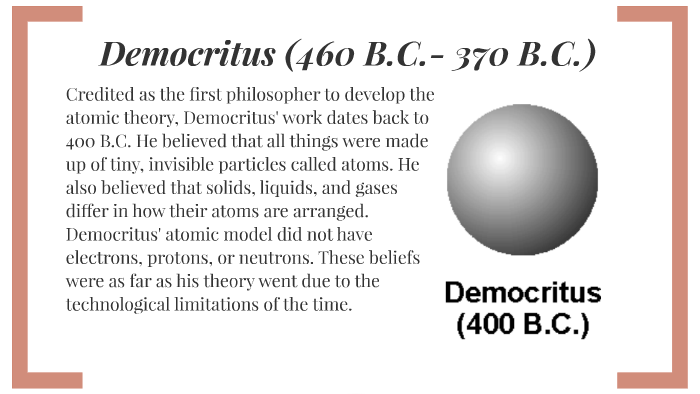
Democritus, theorized that atoms were specific to the material which they composed. In addition, Democritus believed that the atoms differed in size and shape, were in constant motion in a void, collided with each other; and during these collisions, could rebound or stick together. An atomic model is a way of represent the structure of atoms, which in turn tries to explain the way in which they behave and their properties, throughout the entire history of mankind, there have been several of these models, but the first philosopher to postulate one of these was Democritus... Models of the Atom: a Historical Perspective. Early Greek Theories. • 400 B.C. - Democritus thought matter could not be divided indefinitely. • This led to the idea of atoms in a void. • 1800 -Dalton proposed a modern atomic model based on experimentation not on pure reason.
Democritus atomic model diagram. The atomic model of Democritus was the first model of philosophical atomism to try to explain the constitution of materials. Democritus affirmed that the number of atoms is infinite, they are not created and are eternal, and the qualities of an object depend on the types of atoms that compose it. In his model, atoms are homogeneous. democritus atomic model date. › Verified Just Now. Details: I recommend the following article to answer your question. An excerpt is below: A History of Muslim Philosophy Ash'arite Atomism ‑ The substances perceived by us are atoms which come into... Atomic model of Democritus. For many years, man has wondered how matter is formed . Matter, which is a fundamental aspect in the area of chemistry , is composed of small particles which we all know by the name of atoms , which can have specific behavior and properties . Hi, and welcome to this review of atomic models! Today, we're going to be discussing the atomic model and the experiments that led to its He called them atomos, meaning "indivisible," which is where we get our modern word atom. A few things to note about his work: Democritus was a...
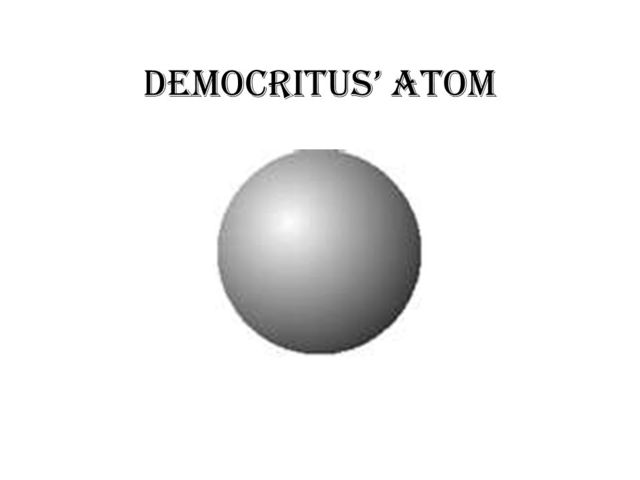
democritus' atomic model. Democritus thought that the atom was just one little sphere. he thought that in between atoms, there was empty space. Democritus' contributions. He was a philosipher, not scienctist. he said atoms are impossible to divide, and impossible to destroy. Democritus' model of an atom was one of an inert solid that interacted mechanically with other atoms. Diogenes Laertius summarized Democritus atomic theory as follows in Lives and Opinions of Eminent Philosophers: "That atoms and the vacuum were the beginning of the universe; and that... Atomic Model. Analogy. Democritus, a philosopher in ancient Greece, began the search for a description of matter. Sep 4, 2009 - Democritus is credited with coming up with the atom. The question Here is a diagram of his experiment. If you shoot Rutherfords experiment prompted a... Democritus' atomic theory. For centuries it was widely believed that matter was made from Earth, air, fire, and water. But, Democritus came up with a theory that explains that atoms exist individually and they combine together to form matter. Dalton' atomic theory.
The atomic model of Democritus He was the first to introduce the idea that matter is made up of indivisible basic elements, called "atoms". The way in which Democritus conceived his model of the atom is far from the current scientific method. One of the philosophical currents of Ancient Greece... Atomic Model s. Democritus (460—370 BC). • Greek Philosopher • Atomism • Nothing exists but atoms. Democritus Atomic Theory. 1. All matter consists of INVISIBLE PARTICLES…..called atoms. 2. Atoms are INDESTRUCTIBLE & UNCHANGEABLE. He Democritus atomic model Is a theory that seeks to explain the structure and representation of atoms and their behavior from logical reasoning and philosophical principles. This model is extracted from the work Atomic Theory of the Universe Conceived by Leucipo but developed by the... Democritus ' Atomic Theory. He believed that atoms were indivisible and indestructible. His theory had some flaws. What were they? He DID not use the scientific method!!. Aristotle. Impact on western civilization Believed all matter was continuous Slideshow 6008579 by xanthus-adkins.
Democritus atomic model was a philosophical model built to solve the problems to change exposed by Parmenides, and most importantly by his disciple Zenon, with his Today the term "atomic model" refers to the structure of the atom (nucleus, electrons,..) Democritus' thinking did not go to this level.
In Democritus philosophy, atoms exist not only in matter, but also in properties such as perception and the human soul. Differences in atomic shape and size determine different properties of matter. Changes in matter are the result of dissociation or combination of atoms as they move through the void.
Democritus Atom Model Diagram - Wiring Diagrams. 6 hours ago Democritus Atom Model Diagram. Democritus was an Ancient Greek pre-Socratic philosopher primarily remembered today for his formulation of an atomic.
Atomic Model of Democritus. Muhammad ShoaibAugust 20, 2020. In addition, Democritus believed that atoms differed in size and shape, were constantly moving in a vacuum, colliding with each other; and during these collisions, they could bounce or stay together.
Learn about democritus atomic model with free interactive flashcards. Choose from 500 different sets of flashcards about democritus atomic model on Quizlet.
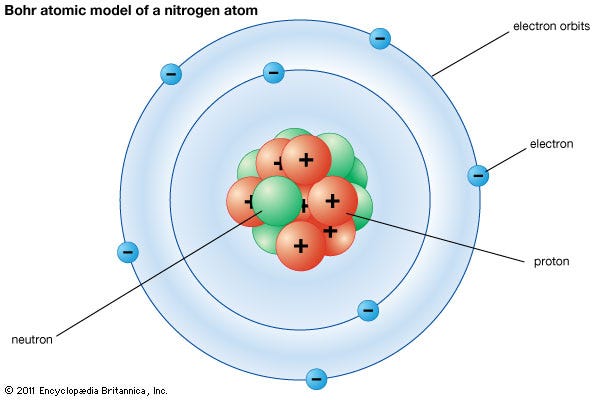
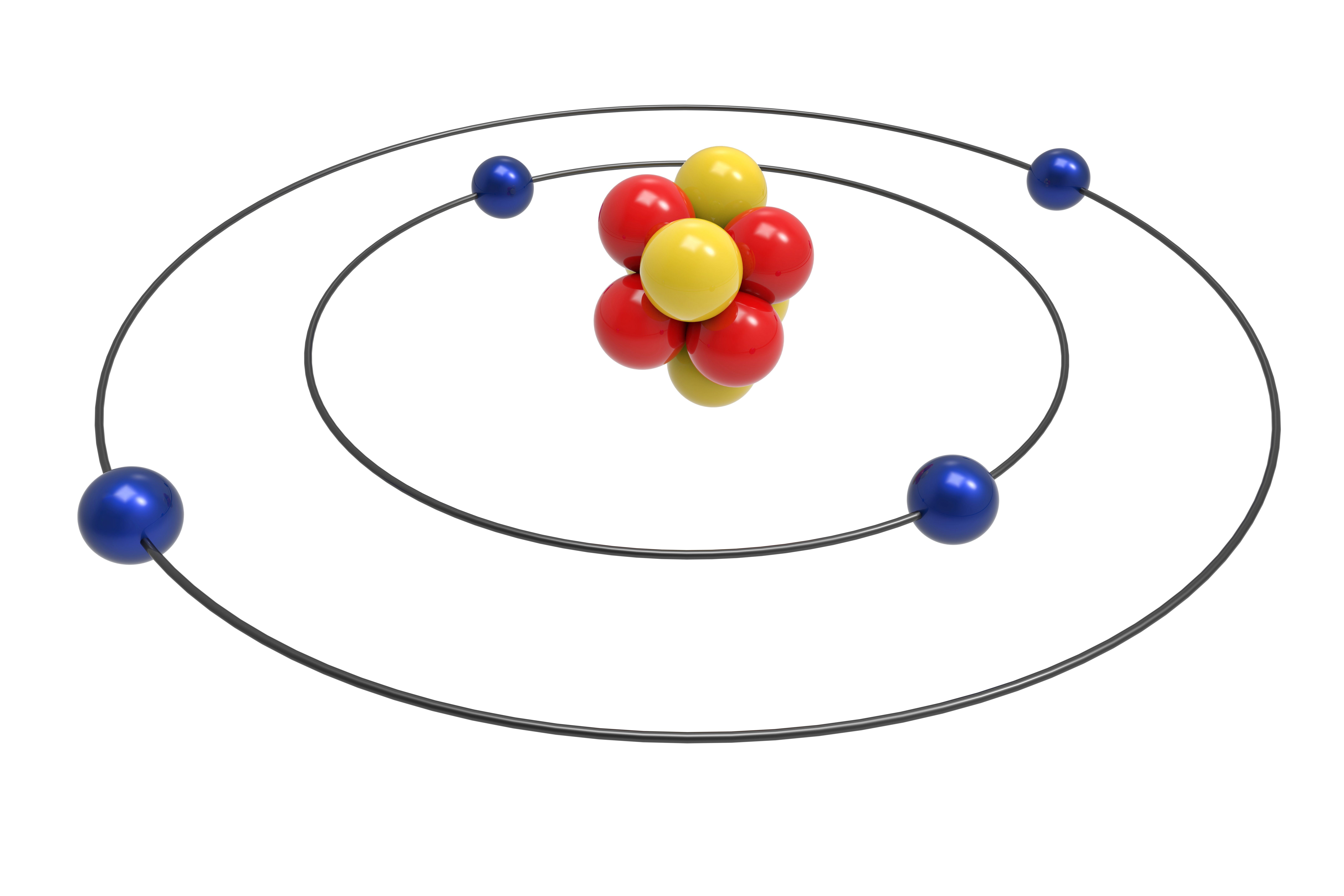
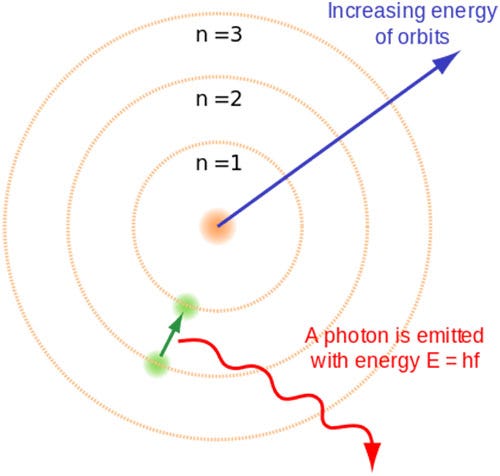
The Atomic Model Worksheet and Key 3. The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. Investigate the contributions of Democritus and John Dalton to the atomic model that Thomson used.
Democritus is credited with coming up with the atom. The question was, what would happen if you keep taking something (like a tree) and breaking Rutherford's experiment prompted a change in the atomic model. If the positive alpha particles mostly passed through the foil, but some bounced back.

Atomic Theory Democritus The Greek Philosopher Democritus 460 B C 370 B C Was Among The First To Suggest The Existence Of Atoms From The Greek Ppt Download
Democritus was an Ancient Greek pre-Socratic philosopher primarily remembered today for his formulation of an atomic theory of the universe. Democritus was born in Abdera, Thrace, around 460 BC, although there are disagreements about the exact year.
7 References. Democritus' atomic theory[change | change source]. Democritus thought that if you cut something in half again and again, you would at last have According to the assumptions established about the atoms neutral charge, Thomson proposed the first atomic model, that was described as a...
Democritus theory of atoms successfully motivated other scientists to conduct other experiments and researches in atomic field. He also stated that every atom is similar to each other which means that atom has no internal structure. The atomic model of Democritus theory is in solid form.
Democritus's model stated that matter consists of invisible particles called atoms and a void (empty space). He stated that atoms are indestructible and unchangeable. His atomic model was solid, and stated all atoms differ in size, shape, mass, position and arrangement, with a void exists between them.
The model that Democritus created contributes to the current model of the atom because it was the. Democritus contributed to the atomic structure by building its foundation. This is because he. conceived the idea of atoms so that scientists could later add the parts ; the shells and nucleus and.
Models of the Atom: a Historical Perspective. Early Greek Theories. • 400 B.C. - Democritus thought matter could not be divided indefinitely. • This led to the idea of atoms in a void. • 1800 -Dalton proposed a modern atomic model based on experimentation not on pure reason.
An atomic model is a way of represent the structure of atoms, which in turn tries to explain the way in which they behave and their properties, throughout the entire history of mankind, there have been several of these models, but the first philosopher to postulate one of these was Democritus...
Democritus, theorized that atoms were specific to the material which they composed. In addition, Democritus believed that the atoms differed in size and shape, were in constant motion in a void, collided with each other; and during these collisions, could rebound or stick together.

How Can We Explain The Structure Properties And Interactions Of Matter Physical Property Physical Change Chemical Property Chemical Change Pure Substance Ppt Download
Atomic Theory Png Democritus Atomic Theory John Dalton Atomic Theory Experiment Thomson Atomic Theory Model John Dalton Atomic Theory Model Name Atomic Theory Powerpoints Animated Atomic Theory Models Did Atomic Theory John Dalton Contribution To

But Wait There S More Atomic Theory Timeline And Periodic Table Notes Science February March 2019 2020 Diagram Quizlet


















0 Response to "39 democritus atomic model diagram"
Post a Comment