39 choose the correct energy diagram describing the lyman and paschen series.
Balbharati solutions for Physics 12th Standard HSC Maharashtra State Board chapter 15 (Structure of Atoms and Nuclei) include all questions with solution and detail explanation. This will clear students doubts about any question and improve application skills while preparing for board exams. The detailed, step-by-step solutions will help you understand the concepts better and clear your ... Other articles where Lyman series is discussed: ionosphere and magnetosphere: Photon absorption: (The Lyman series is a related sequence of wavelengths that describe electromagnetic energy given off by energized atoms in the ultraviolet region.) Lyman α emissions are weakly absorbed by the major components of the atmosphere—O, O2, and N2—but they are absorbed readily by NO and have…
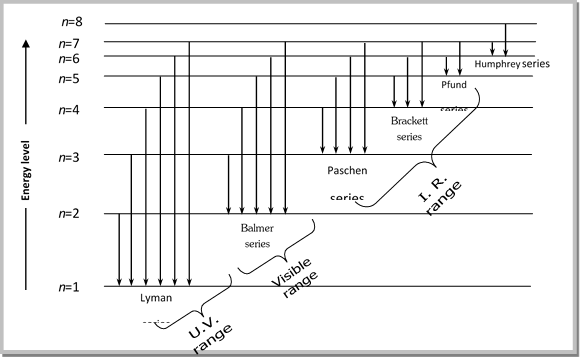
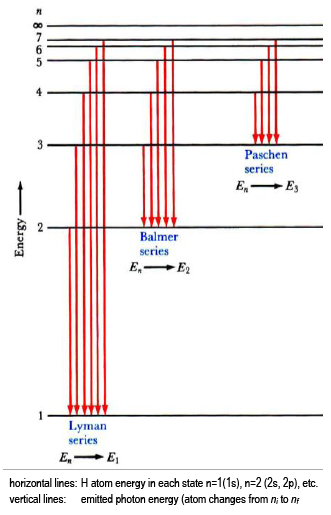
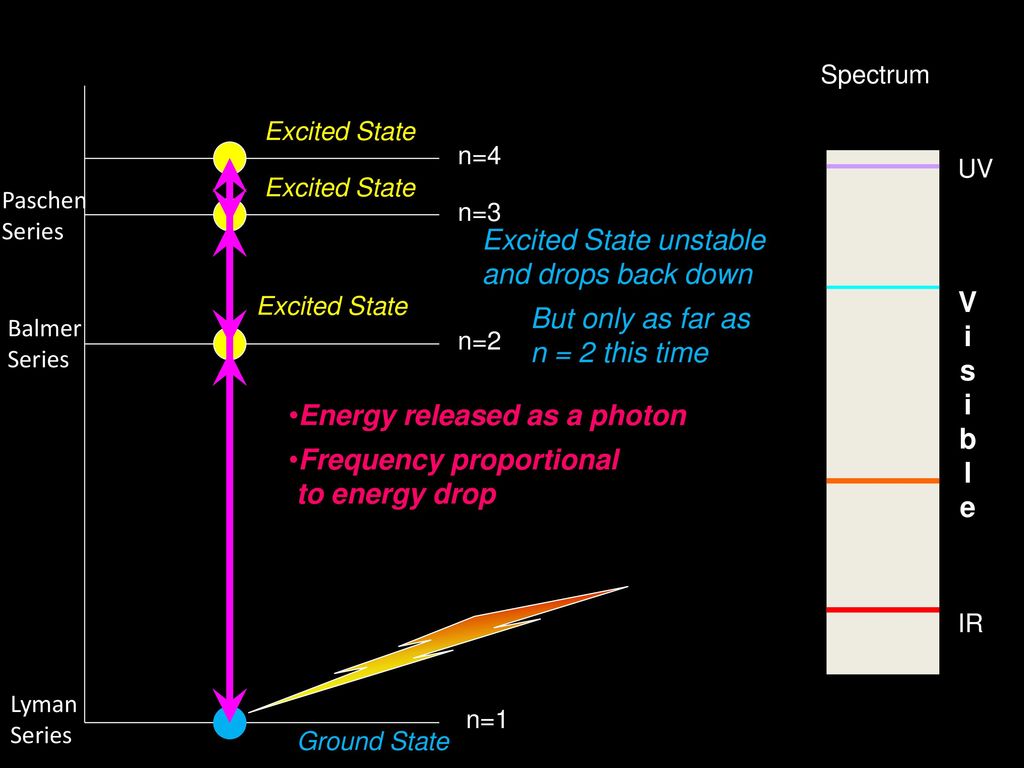
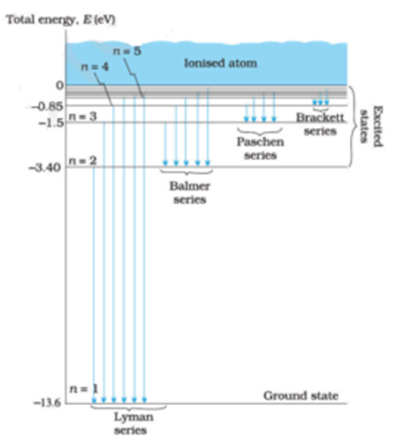
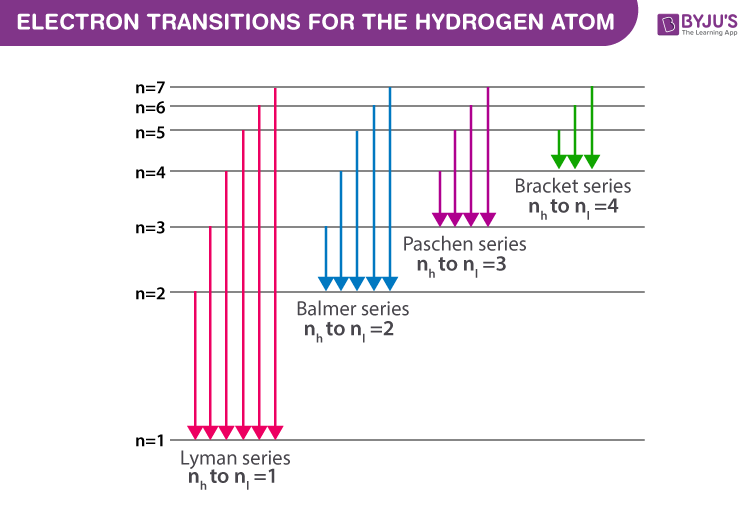
Electron transitions down to level 1 are part of the Lyman series. Transitions down to level 2 are part of the Balmer series. And transitions down to level 3 are known as the Paschen series. When we look at the remaining answer options, we see that option (D) shows transitions to the third energy level, which is the Paschen series.
Choose the correct energy diagram describing the lyman and paschen series.
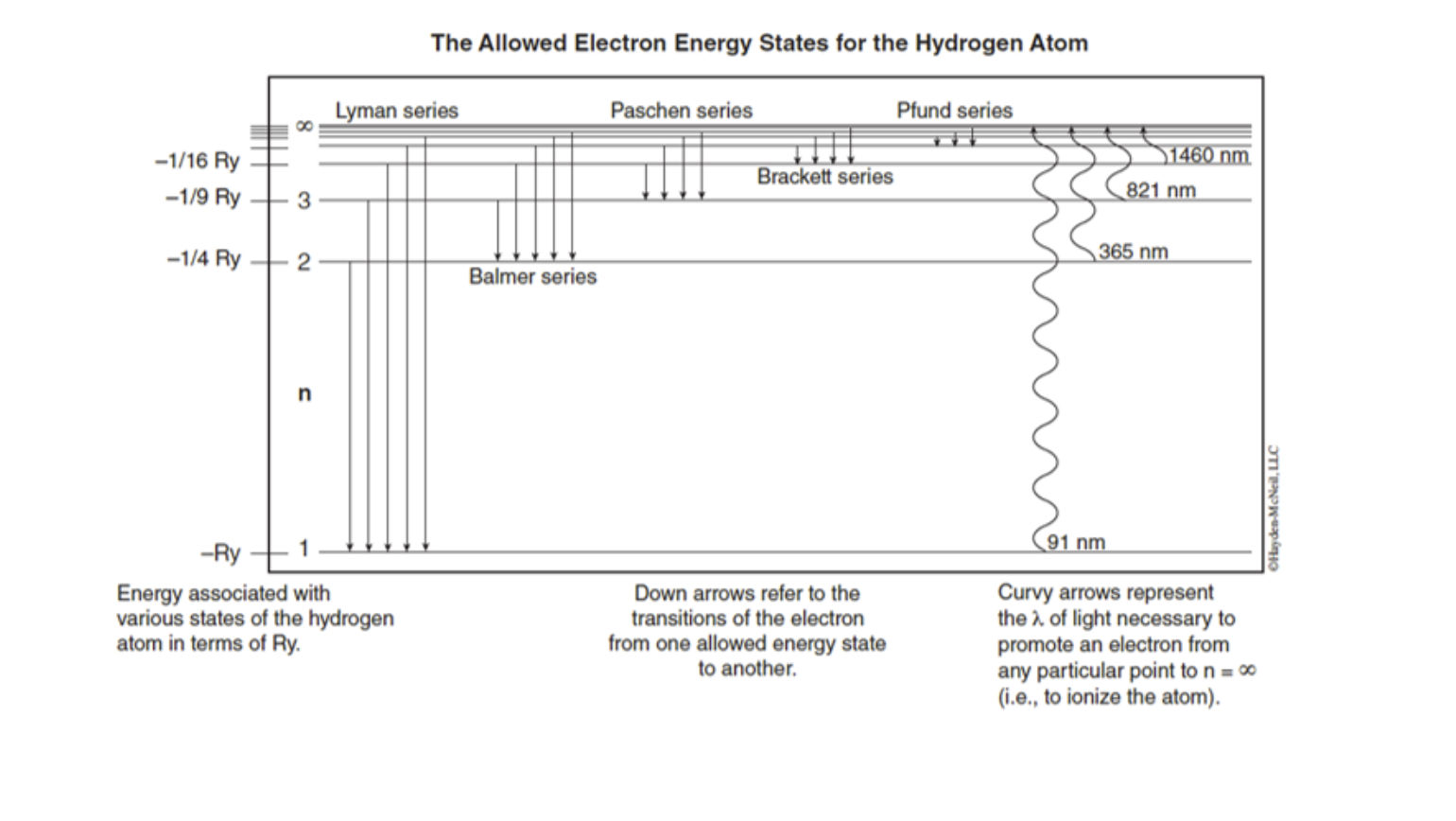
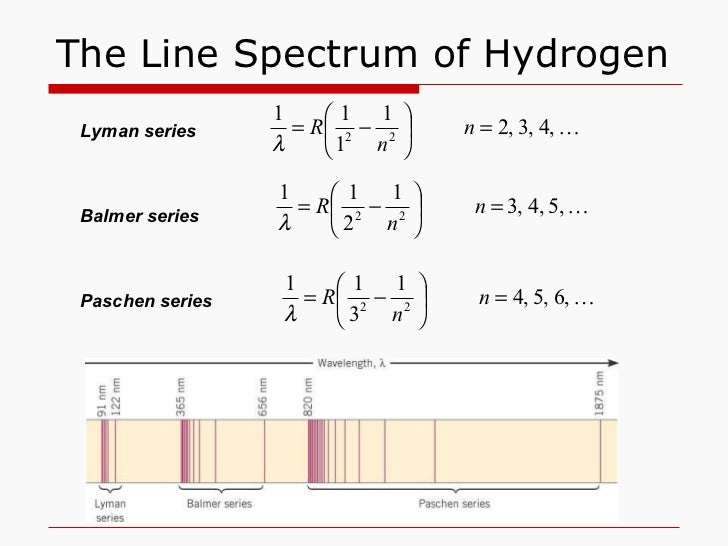
Paschen series (n l =3) The series was first observed during the years 1908, by a German physicist Friedrich Paschen. Thus the series is named after him. Paschen series is displayed when electron transition takes place from higher energy states(n h =4,5,6,7,8,…) to n l =3 energy state. We get a Lyman series of the hydrogen atom. It is obtained in the ultraviolet region. This formula gives a wavelength of lines in the Lyman series of the hydrogen spectrum. Different lines of Lyman series are. α line of Lyman series p = 1 and n = 2. α line of Lyman series p = 1 and n = 3. γ line of Lyman series p = 1 and n = 4. Further, for n=∞, you can get the limit of the series at a wavelength of 364.6 nm. Also, you can't see any lines beyond this; only a faint continuous spectrum.Furthermore, like the Balmer's formula, here are the formulae for the other series: Lyman Series. Paschen Series. Brackett Series. Pfund Series
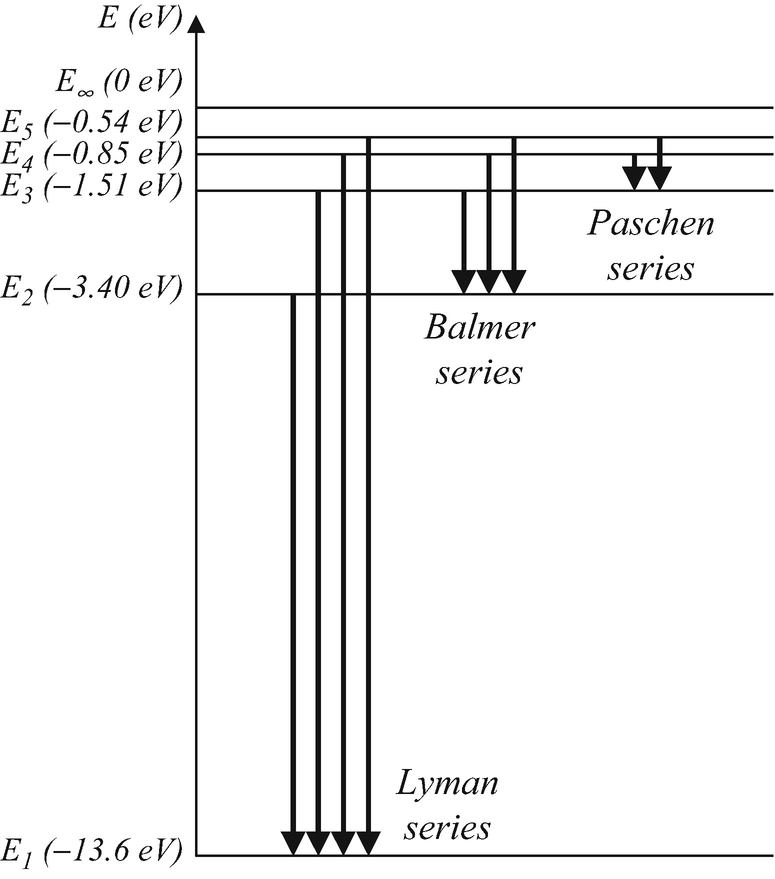
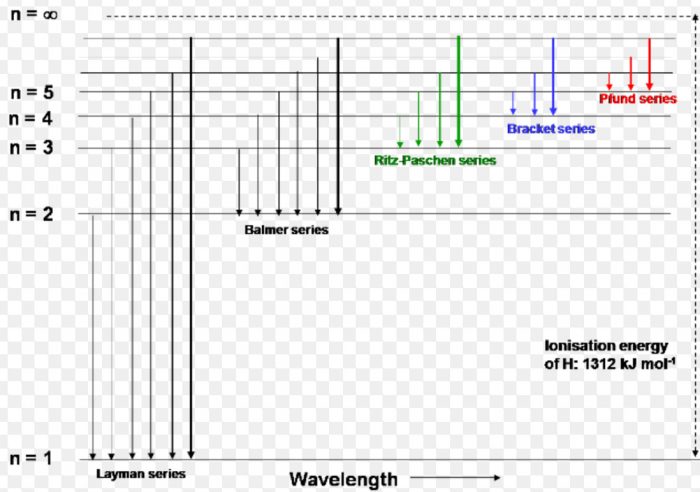
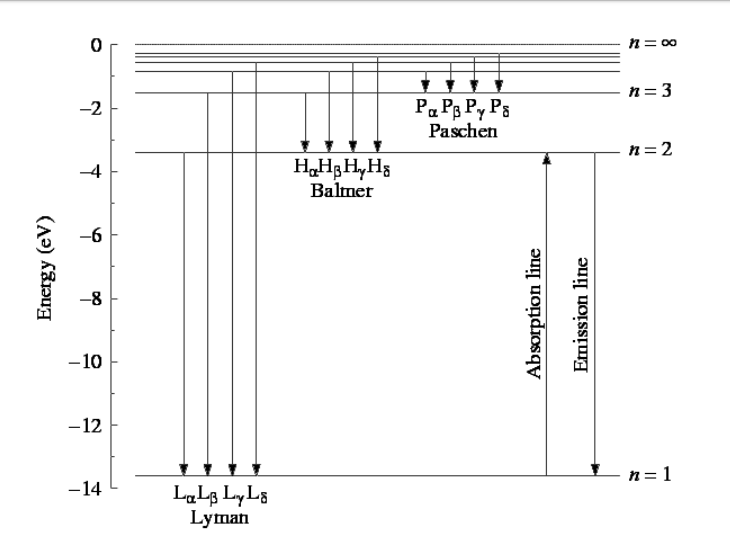
Choose the correct energy diagram describing the lyman and paschen series.. In physics and chemistry, the Lyman series is a hydrogen spectral series of transitions and resulting ultraviolet emission lines of the hydrogen atom as an electron goes from n ≥ 2 to n = 1 (where n is the principal quantum number), the lowest energy level of the electron. PHYS 1493/1494/2699: Exp. 7 - Spectrum of the Hydrogen Atom 2 Introduction The physics behind: The spectrum of light The empirical Balmer series for Hydrogen The Bohr model (a taste of Quantum Mechanics) Brief review of diffraction The experiment: How to use the spectrometer and read the Vernier scale Part 1: Analysis of the Helium (He) spectrum Energy-level diagram for hydrogen showing the Lyman, Balmer, and Paschen series of transitions. The orbital energies are calculated using the above equation, first derived by Bohr. Electron total energies are negative, since the electron is bound to the nucleus, analogous to being in a hole without enough kinetic energy to escape. Energy-level diagram for hydrogen showing the Lyman, Balmer, and Paschen series of transitions. The orbital energies are calculated using the above equation, first derived by Bohr. Figure 7 shows an energy-level diagram for hydrogen that also illustrates how the various spectral series for hydrogen are related to transitions between energy levels.
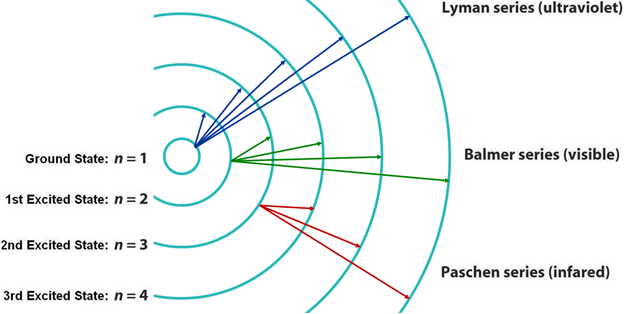
The Lyman series lines occur in the ultraviolet region, the Balmer are in the visible region, and the Paschen and Brackett are in the infrared. The wavelength of the photons emitted by the hydrogen atom may also be calculated using the Rydberg equation shown below. Lyman, Balmer, and Paschen series. o Clicking on the label will shoot a photon of that energy. o If the photon is in visual band, its true color is shown. Photons of longer wavelengths are shown as red and shorter wavelengths as violet. ? The ?Event Log? in the lower right lists all the photons that the atom has encountered as well as all the ... The transitions, which are responsible for the emission lines of the Balmer, Lyman, and Paschen series, are also shown in Fig. 1.6. The Balmer emission lines correspond to transitions from the levels for which n is greater than or equal to 3 down to the level for which n = 2. These transitions all produce light in the visible part of the spectra. A. Choose the correct alternative (a), (b), (c) or (d) for each of the questions given below: ... Wavelengths of the first lines of the Lyman series, Paschen series and Balmer series, in the hydrogen spectrum are denoted by λL, λP, and λB, respectively. ... Correct diagram with at least one arrow, an incident or reflected ray.
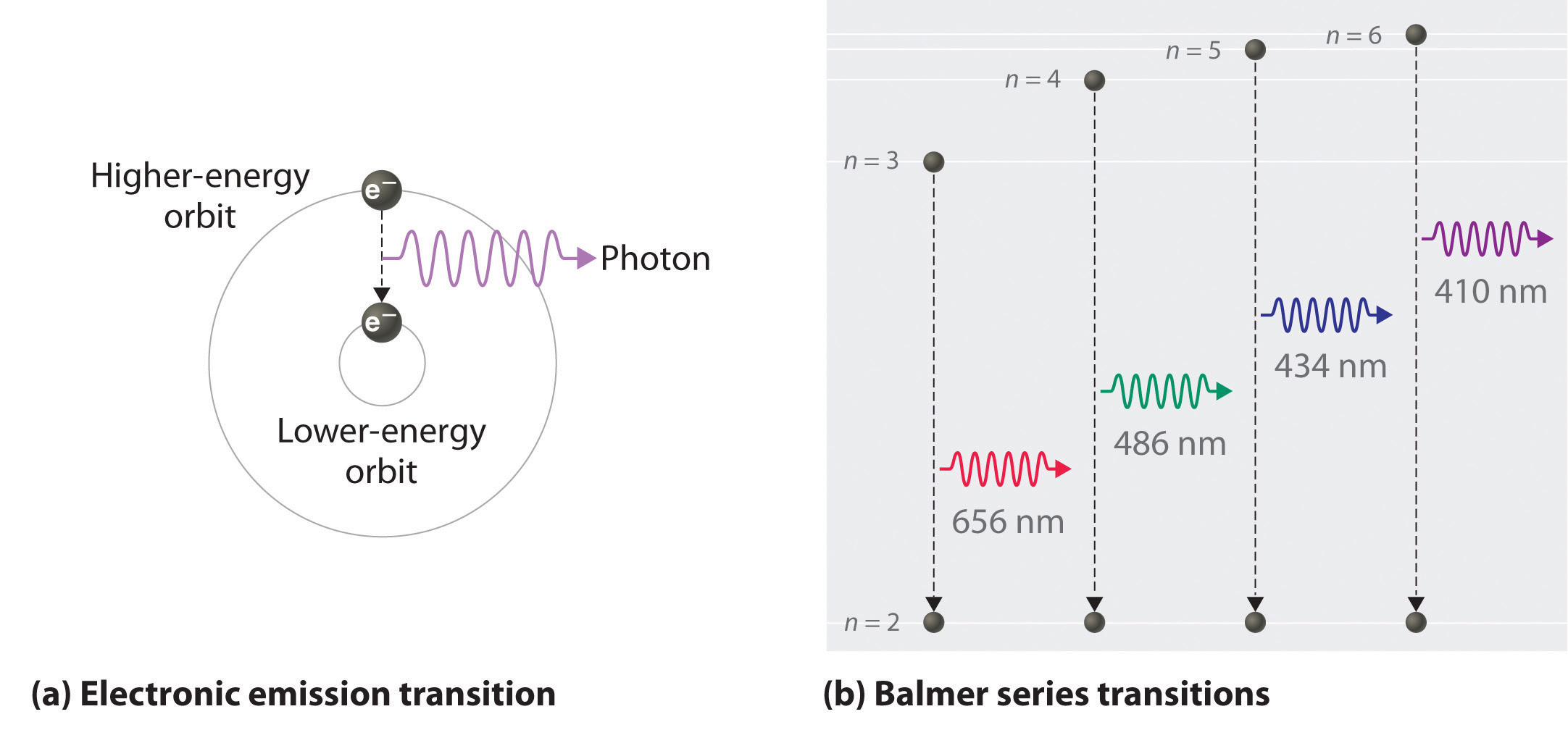
The Balmer series, or Balmer lines in atomic physics, is one of a set of six named series (Lyman, Balmer, Paschen, Brackett, Pfund) describing the spectral line emissions of the hydrogen atom, that result from electron transitions from higher levels down to the energy level with principal quantum number 2. [AL] Have a student draw a planetary model to match the energy-level diagram shown above. Have students model the changes in energy states on their planetary model as they are shown on the energy-level diagram. Encourage students to use terminology such as Balmer series, Lyman series, and Paschen series in their explanations. The diagram below shows three of these series, but there are others in the infra-red to the left of the Paschen series shown in the diagram. The diagram is quite complicated, so we will look at it a bit at a time. Look first at the Lyman series on the right of the diagram - this is the most spread out one and easiest to see what is happening. The Balmer series includes the lines due to transitions from an outer orbit n > 2 to the orbit n' = 2. Named after Johann Balmer, who discovered the Balmer formula, an empirical equation to predict the Balmer series, in 1885. Balmer lines are historically referred to as "H-alpha", "H-beta", "H-gamma" and so on, where H is the element hydrogen.Four of the Balmer lines are in the technically ...
atom that have been measured. The Lyman series is a set of ultraviolet lines that fit the relationship with ni = 1. A series in the infrared region of the spectrum is the Paschen series that corresponds to ni = 3. The Brackett and Pfund series are two more in the infrared region corresponding to ni = 4 and ni = 5.
Energy-level diagram for hydrogen showing the Lyman, Balmer, and Paschen series of transitions. The orbital energies are calculated using the above equation, first derived by Bohr. Electron total energies are negative, since the electron is bound to the nucleus, analogous to being in a hole without enough kinetic energy to escape.
Correct option is . D. Balmer series. Lyman series of hydrogen atom lies in ultraviolet region, Balmer series lies in visible region while Pfund and Paschen series lie in infrared region. Video Explanation. Was this answer helpful? 0. 0. Similar questions.
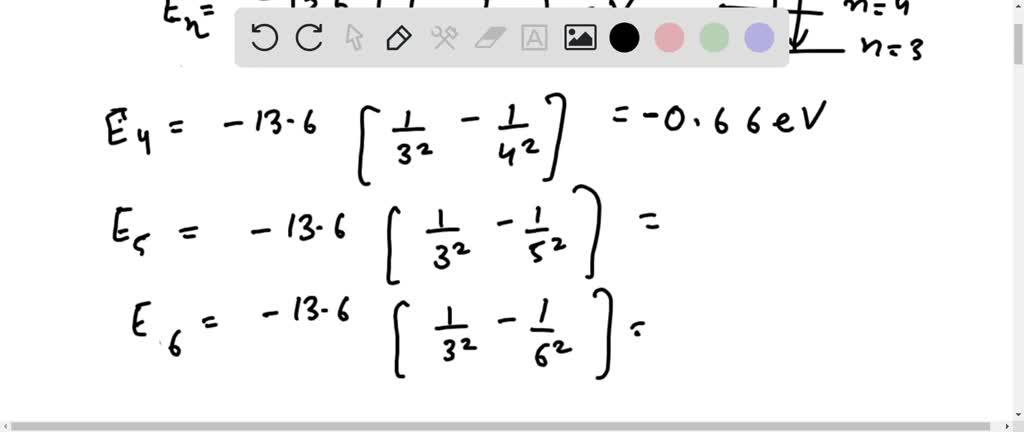
Solved Find The Energy Associated With The 4th Line Of The Paschen Series In The Hydrogen Emission Spectrum Answer Choices 1 29 Ev 1 34 Ev 1 23 Ev 1 13 Ev
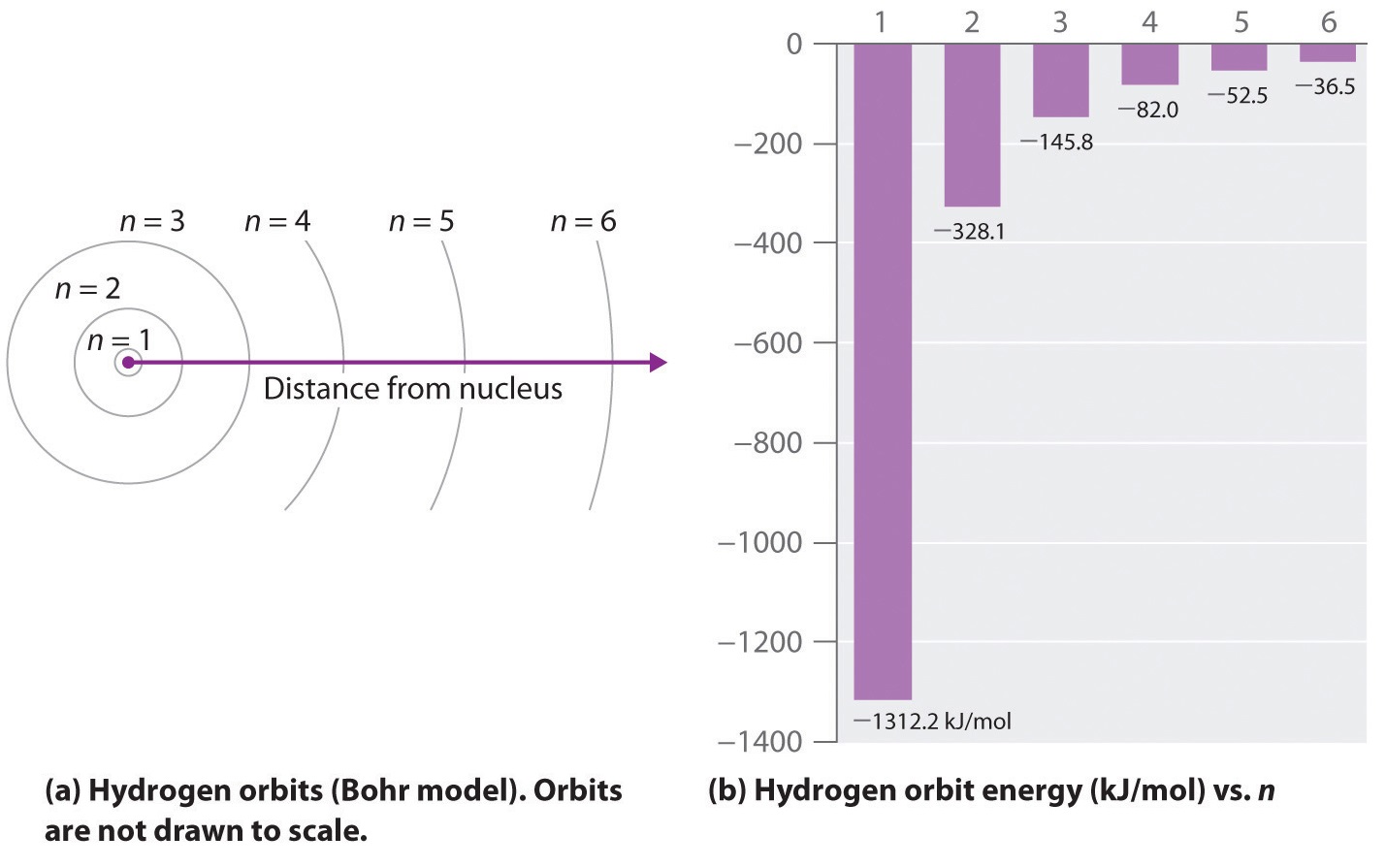
Figure 2: Energy-Level Diagram for Hydrogen and the Bohr Model for Hydrogen. The right hand side (a) of the figure shows the Bohr model with the Lyman, Balmer, and Paschen series illustrated. A small circle representing the nucleus is enclosed by a larger circle for orbit n = 1, then another larger circle for n = 2 and so on up to n = 5.
the amount of energy needed to move an electron from one energy level to another (so... the energy of an electron is said to be quantized) ... a mathematical expression describing the probability of finding an electron at various locations around the nucleus. ... Lyman series 2) Balmar series 3)Paschen series. Lyman series. lines at the ...
The energy diagram for hydrogen is shown in Fig. 1. Vertical arrows indicate transitions between energy levels. During each transition a photon of frequency f = (Ei - Ef)/h is emitted. 2. Procedure You will measure the spectra using USB4000 spectrometer. The spectrometer measures the amount of light of each wavelength in the sampled spectrum.
Q5: Explain why Lyman and Paschen series absorption lines are not seen in each panel's spectrum. Q6: In the top graph of each panel of spectra (starting on the next page), circle each of the 3 Balmer series absorption lines you drew on the energy level diagram in the first
The energy difference between the various levels of Bohr's model and the wavelengths of absorbed or emitted photons is represented by the Rydberg formula. It can be stated mathematically as- 1/λ = RZ 2 (1/n 12 − 1/n 2h) There are six types of spectral series- Lyman, Balmer, Paschen, Brackett, Pfund, and Humphreys.
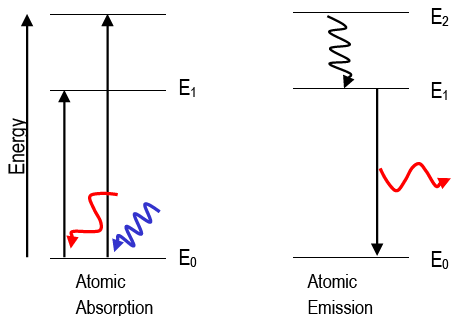
In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are "captured ...
First member of Lyman series, third spectral line of Balmer series and the second spectral line of Paschen series done clear. B) ... The figure indicates the energy level diagram of an atom and the origin of six spectral lines in emission (e.g. line no. 5 arises from the transition from level B to A). ...
Lyman Series. When an electron jumps from any of the higher states to the ground state or 1st state (n = 1), the series of spectral lines emitted lies in ultra-violet region and are called as Lyman Series. The . wavelength (or wave number) of any line of the series can be given by using the relation: = RZ 2 (1/1 2 - 1/n 2 2), n 2 = 2, 3, 4, 5
Jee Main Jee Advanced Cbse Neet Iit Free Study Packages Test Papers Counselling Ask Experts Studyadda Com
Further, for n=∞, you can get the limit of the series at a wavelength of 364.6 nm. Also, you can't see any lines beyond this; only a faint continuous spectrum.Furthermore, like the Balmer's formula, here are the formulae for the other series: Lyman Series. Paschen Series. Brackett Series. Pfund Series
We get a Lyman series of the hydrogen atom. It is obtained in the ultraviolet region. This formula gives a wavelength of lines in the Lyman series of the hydrogen spectrum. Different lines of Lyman series are. α line of Lyman series p = 1 and n = 2. α line of Lyman series p = 1 and n = 3. γ line of Lyman series p = 1 and n = 4.
Paschen series (n l =3) The series was first observed during the years 1908, by a German physicist Friedrich Paschen. Thus the series is named after him. Paschen series is displayed when electron transition takes place from higher energy states(n h =4,5,6,7,8,…) to n l =3 energy state.

Please Explain Lyman Balmer Paschen And Brackett Series Chemistry Structure Of Atom 4885537 Meritnation Com

A Draw The Energy Level Diagram For The Line Spectra Representing Lyman Series And Balmer Series In The Spectrum Of Hydrogen Atom B Using The Rydberg Formula For The Spectrum Of Hydrogen

Wavelengths Of The First Lines Of The Lyman Series Paschen Series And Balmer Series In Hydrogen Spectrum Are Denoted By Lambda L Lambda P And Lambda B Respectively Arrange These Wavelength In

Write The Different Types Of Hydrogen Spectral Series The Lyman Series Of Hydrogen Spectrum Lies In The Ultraviolet Region Why













0 Response to "39 choose the correct energy diagram describing the lyman and paschen series."
Post a Comment