38 lewis dot diagram for co
Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen. That is carbon contributes its 2 electrons and oxygen contributes its two electrons to form a double bond . Now carbon is left with 2 valence electrons and it ...3 answers · 13 votes: Usually shown as There are various ways of configuring the electrons. Formal charge Suggests ...
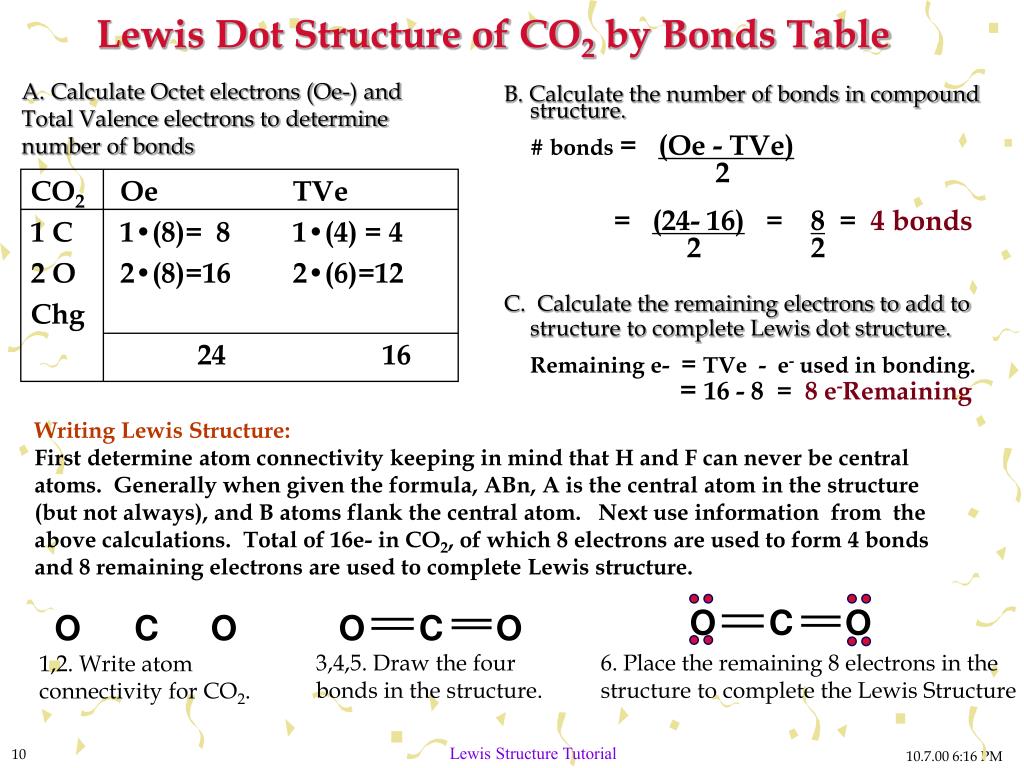
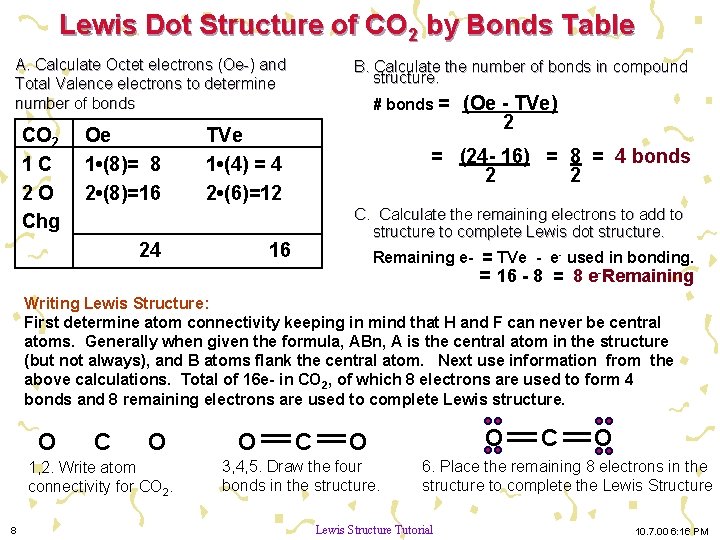
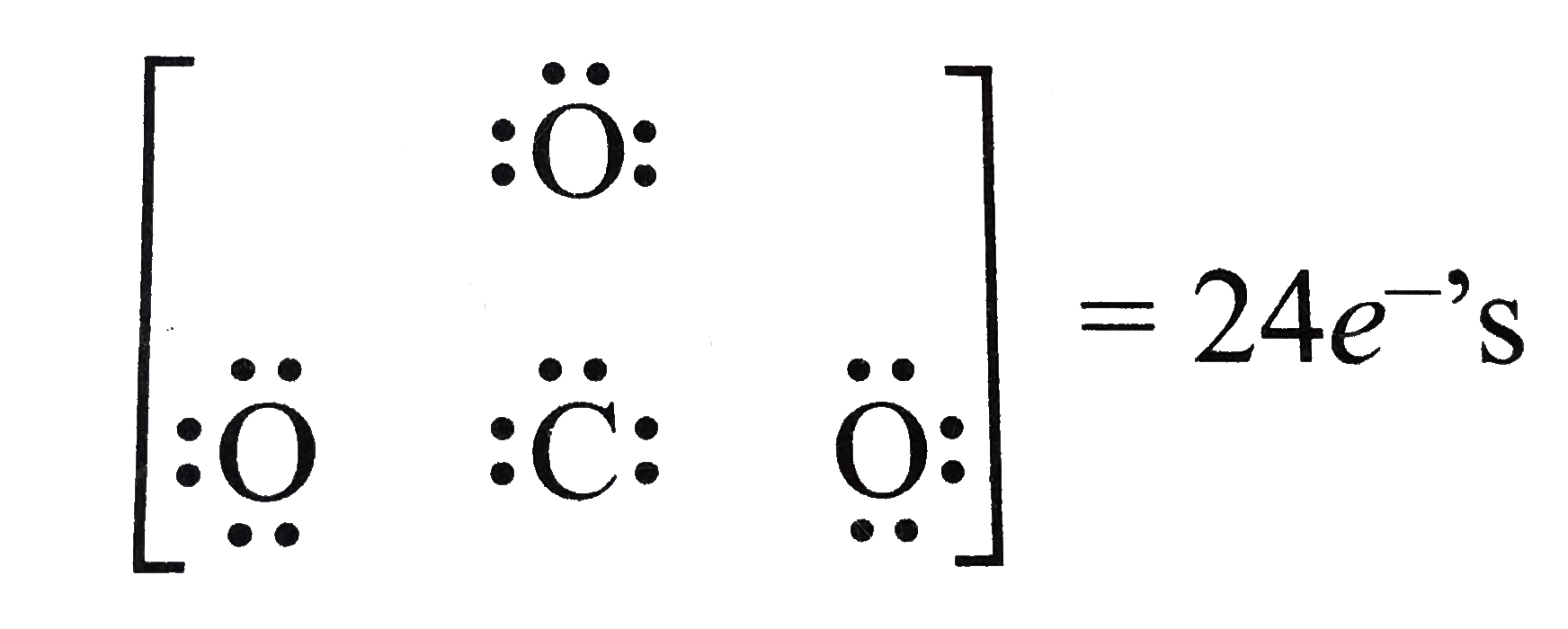
2 weeks ago - CO2 Lewis Structure The CO2 Lewis structure has two double bonds going from carbon to the oxygen atoms. According to the octet rule, each oxygen atom needs to bond twice and the carbon atom needs to bond four times. CO2 Lewis Structure Setup Let’s think in terms of dots to make the CO2 Lewis ...

Lewis dot diagram for co
Lewis Dot Structure Definition. Lewis dot structure definition: a visual way to clearly depict the connection of atoms and the electrons present in a molecule.With a carbon Lewis dot structure, one can see how the atoms in a molecule are bonded together, which gives us more information about the structure than the molecular formula. The former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well. Carbon needs two double bonds, one to each of the two oxygens, to complete its octet. The atoms *share* electrons with each other because they are both non-m...
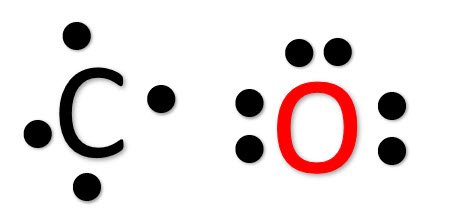
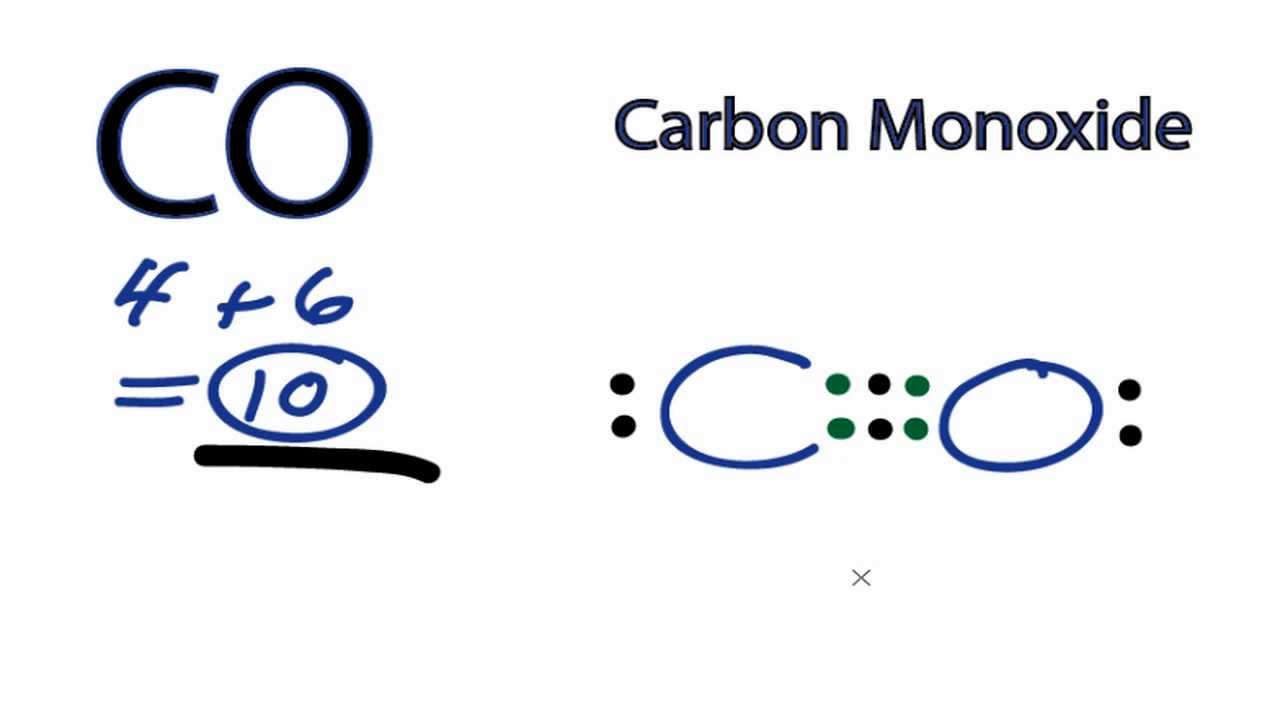
Lewis dot diagram for co. Lewis dot structure of H 2 CO. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. H:1x2=2 C:4 O Total= May 03, · May 6, - Uploaded by Wayne Breslyn. For the CH2O Lewis structure, calculate the total number of valence electrons for the CH2O molecule. The skeletal structure of CO is written as: C O Step 3. Draw a single bond (one shared electron pair) between C and O and complete the octet on O, the remaining two electrons are the lone pair on C. CO Lewis Structure, Geometry, and Hybridization. Carbon monoxide (CO) is a tasteless and odorless flammable gas that is quite toxic in nature to the fauna. It is so because, carbon monoxide uses hemoglobin, an oxygen carrier, to reach throughout the body when in a concentration of more than 35ppm. The carbon monoxide is produced from the ... Do you need help with your homework? On Toppr Answr you can scan any question, and get its answer instantly
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ]. The Lewis Dot Structure is a graphical representation of how electrons are distributed around the atoms which comprise a molecule. The reason for drawing/creating a Lewis dot structure is that it helps one predict the kinds of bonds, as well as a number of bonds, that can be formed around an atom. Lewis structures can be utilized to make ... A step-by-step explanation of how to draw the CH4N2O Lewis Dot Structure (Urea).For the CH4N2O structure use the periodic table to find the total number of v... A step-by-step explanation of how to draw the CO(Carbon Monoxide) Lewis Dot Diagram.For the CO structure use the periodic table to find the total number of v...
For example, CO 2 is a neutral molecule with 16 total valence electrons. In the Lewis structure, carbon should be double-bonded to both oxygen atoms. The Lewis structure for carbon dioxide. This diagram shows the conceptual stages of drawing the Lewis structure for a molecule of carbon dioxide (CO2). Lewis structures can also be drawn for ions. Lewis Electron Dot Structure for the molecule: CO 2. An oxygen atom has 6 valence electrons and a carbon atom has 4. So carbon shares 2 with one oxygen atom and 2 with the other oxygen atom. Hence two double bonds are formed. Lewis Electron Dot Structure of the molecule: CO (carbon monoxide) The carbon atom has a valency of 4. Lewis dot structure of H 2 CO. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. H:1x2=2 C:4 O Total= b Draw the Lewis structure for H2CO. By knowing the Lewis structure, we can also predict the three-dimensional geometry of an individual molecule. Electron dot structures or Lewis dot formula – It defines the nature of bond and position of atoms of the molecule which are connected in the molecule.
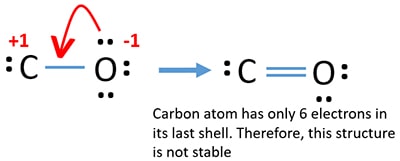
October 30, 2015 - This often looks wrong to a student who is used to seeing double bonds on oxygen. Students are typically taught an electron-counting method, which goes as follows: Count the number of valence electrons per atom. Draw out a predicted atom connectivity. Place all electrons in predicted spots.
- Lewis dot structure or electron dot structure or sometimes also called as Lewis electron dot structure is the diagram which shows the bonding between the atoms of a molecule and the lone pair of electrons that may exist in the molecule. - Lewis structure shows each tom and its position in the structure of a molecule by making use of chemical ...
The Lewis Dot Structure for CO2. Created by MakeTheBrainHappy. This is the Lewis Dot Structure for CO2. You could alternatively also draw the structure by including two dots for every bond. That would mean that you would have a total of eight dots around the carbon, thereby filling its octet. The octets of both of the oxygen atoms are also ...
Lewis structures of cyanate, lewis electron dot structure of co2, pi and, electron bonding, college chemistry tutor, lewis structure of CO2, electron dot structures of carbon dioxide CO2, pi bond s, electron dot co2, Lewis electron structures, for the draw, video, CO2, lewis formula of co2, lewis formula of carbon dioxide,Lewis structures of cyanate, lewis electron dot structure of co2, pi and ...
January 8, 2018 - Put least electronegative atom in centre 3. In the earths atomsphere it is considered a greenhouse gas. Dot Generator Reson...
Learning electron configuration and Lewis dot structure, the homework asks for Co-cobalt, witch is a transition metal- anyhow I can't find it :( Unknown008 Posts: 8,076, Reputation: 723. Uber Member : Dec 5, 2011, 12:15 AM Cobalt has atomic number 27 and is found in the 4rd row of the periodic table, to the left of copper and nickel. ...

Solved Draw The Lewis Structure For Co With An Arrow Representing The Dipole Moment Use Figure 5 5 To Estimate The Percent Ionic Character Of The Co Bond
CO Lewis structure, Hybridization, and Molecular Geometry (Carbon Monoxide) Carbon Monoxide is a colorless and odorless gas. This gas is less dense than the air and flammable. People know about this gas, as it can also cause poisoning. Carbon Monoxide is a toxic gas that binds with hemoglobin, which interferes with its binding with Oxygen.
December 17, 2018 - Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us!

Carbon Dioxide Molecule Co2 Lewis Dot Cross Electronic Diagram Covalent Bonds Ball Stick Space Filling 3d Models Boiling Point Melting Point Doc Brown S Chemistry Revision Notes
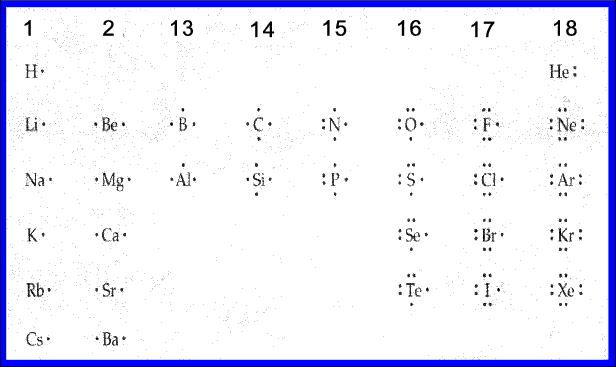
A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
The University of Missouri has announced updated COVID-19 policies that go into effect August 2, 2021. For the very latest information, please refer to https://renewal.missouri.edu/
Ppt Drawing Lewis Structures A Tutorial On Writing Lewis Dot Structure Powerpoint Presentation Id 334634
The lewis dot structure diagram depicts the placement of electrons in the molecules of any compound. Media Portfolio from wps.prenhall.com For carbon, the sixth electron must also go into a 2p orbital; Lewis structures, also known as lewis dot diagrams, lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot ...
The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. YouTube. Wayne Breslyn. 407K subscribers.
Let us consider the case of the Lewis electron dot structures of carbon monoxide CO. Carbon monoxide is an odorless, colorless, non-irritant gas. It is the most common cause of fatal poisoning in Britain today. It causes the accidental deaths of up to 50 persons each year in the U.K. alone and a much larger number of non-fatal poisonings.
April 18, 2015 - 1. 2. Two electrons (dots) make one bond (line)
Complete the Lewis dot structure for IN2 Complete the Lewis dot structure for C02 10. Complete the Lewis dot structure for CO 11. Complete the Lewis dot structure for HCN STOP Your group will check your answers with the instructor before moving on. Model V: Resonance Structures— When One Lewis Structure Isn't Enough Read This!
Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
Lewis Structures: Lewis Structures are essentially dot-diagrams that use single, double, and triple lines to present the shared electrons in a molecule and the guideline is that each atom must ...
In the CO Lewis structure there aren't enough valence electrons available for each atom to obtain an octet without sharing more than one pair. Therefore CO has a triple bond between the carbon and oxygen atom. For the CO Lewis structure there are a total of 10 valence electrons available.
Hint: The atomic number of carbon is 6 and the atomic number of oxygen is 8. - The oxygen can form two single bonds and one double bond with other atoms. Complete step by step answer: So in the question it is asked that how, one can draw the Lewis dot diagram of carbon dioxide.
May 22, 2017 - I realize that explaining how to draw the lewis dot structure in words might get confusing so what I'm going to do is put a picture of what the diagram will look like along with a video showing step by step on how to exactly create the diagram for this specific example.
Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial. CO 2 lewis structure and Shape. In the lewis structure of CO 2, you can see there are two double bonds around carbon atom. Each oxygen atom has two lone pairs and carbon atom does not have a lone pair. Also, there are no charges in oxygen atoms and carbon atom.
February 3, 2021 - Carbon Dioxide is one of the best compounds to start with learning the concepts of Lewis structure and Molecular Geometry. This molecule can be a good start for beginners who want to learn the fundamentals of such concepts and want to know how to draw Lewis dot structures for other molecules ...
Carbon needs two double bonds, one to each of the two oxygens, to complete its octet. The atoms *share* electrons with each other because they are both non-m...
The former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well.
Lewis Dot Structure Definition. Lewis dot structure definition: a visual way to clearly depict the connection of atoms and the electrons present in a molecule.With a carbon Lewis dot structure, one can see how the atoms in a molecule are bonded together, which gives us more information about the structure than the molecular formula.
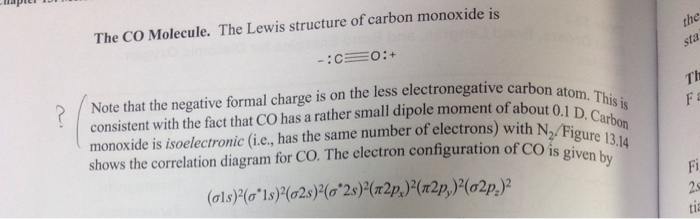


/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)

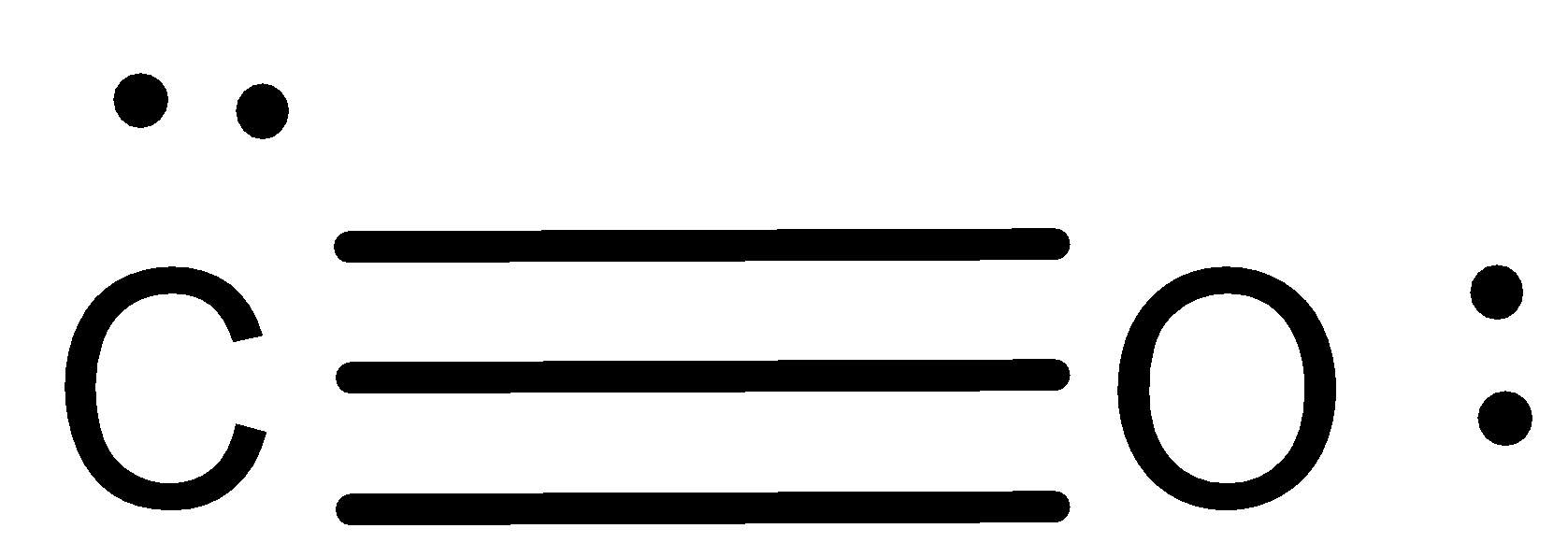

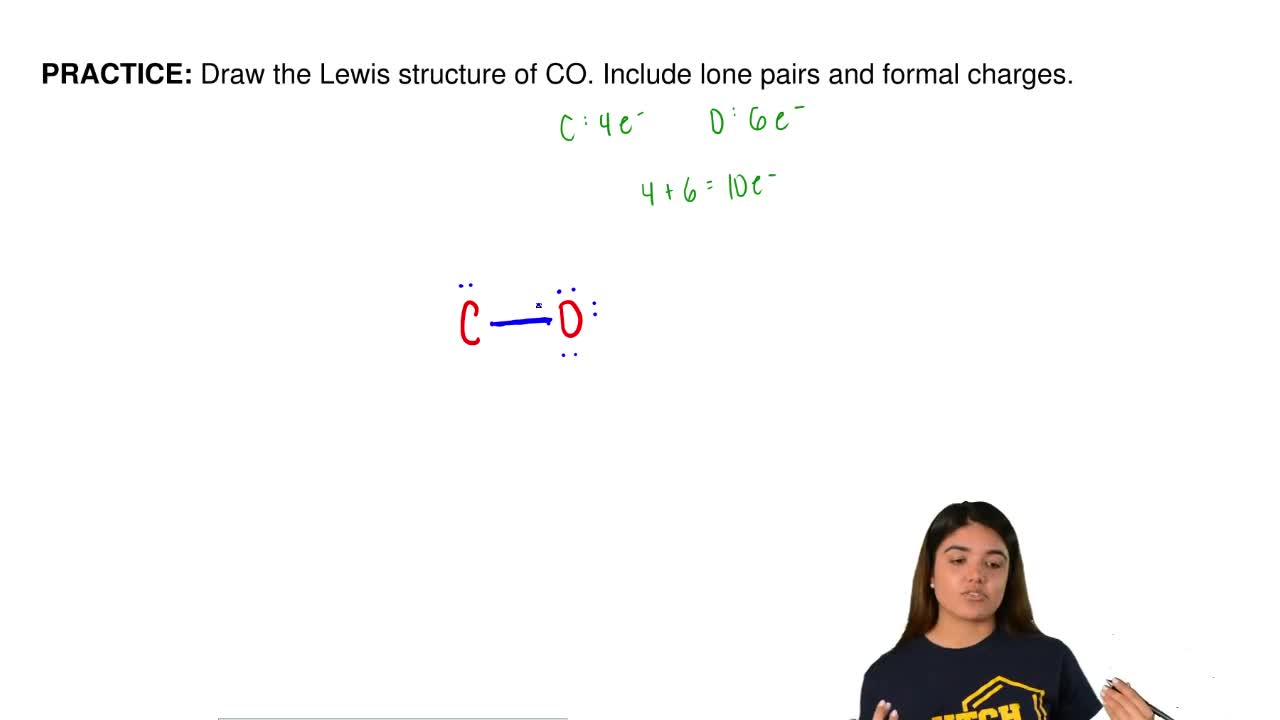




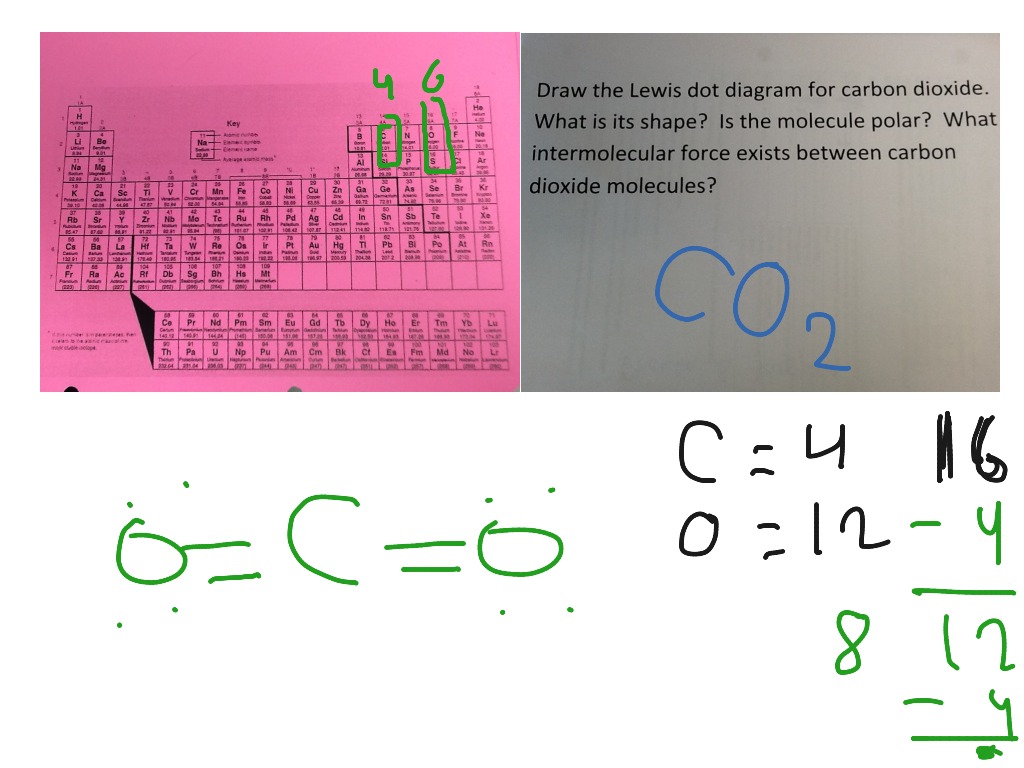


/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)




0 Response to "38 lewis dot diagram for co"
Post a Comment