37 diagram of exothermic reaction
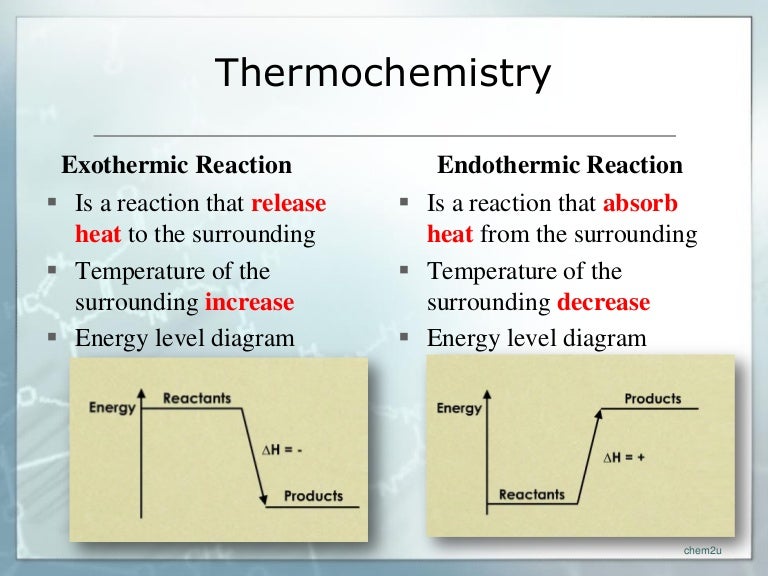
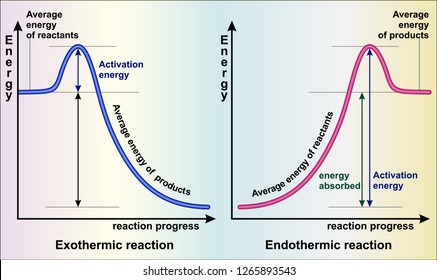
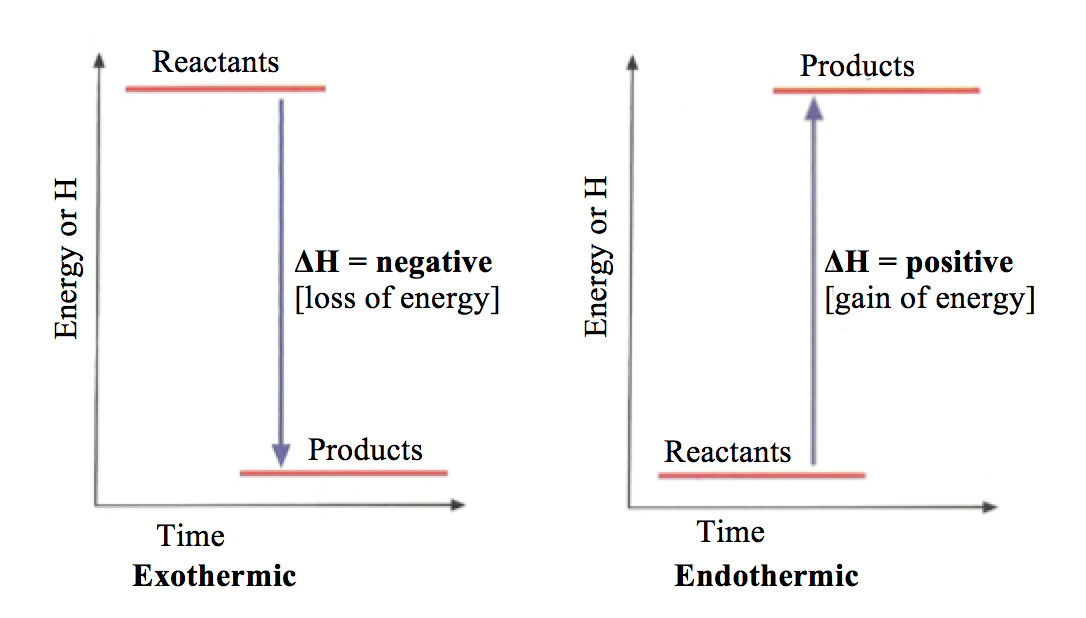
An exothermic reaction is a chemical reaction that releases heat and has a negative enthalpy (-ΔH) and positive entropy (+ΔS).. These reactions are energetically favorable and often occur spontaneously, but sometimes you need a little extra energy to get them started. An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
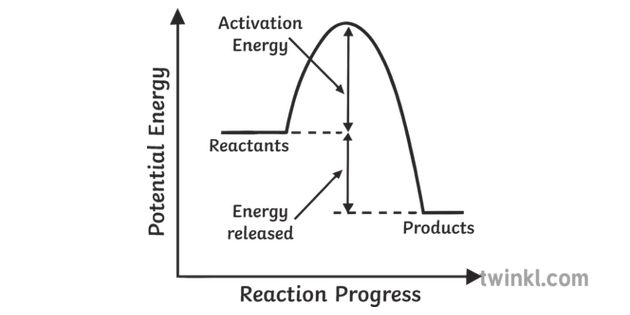
Exothermic Reactions is the flow of the net transfer of heat energy during the reaction is from the medium into its surroundings. In exothermic reactions, the reactants always possess more energy than the products and hence are less stable. For this reason, the exothermic reactions require very less amount of activation energy to initiate the reaction.
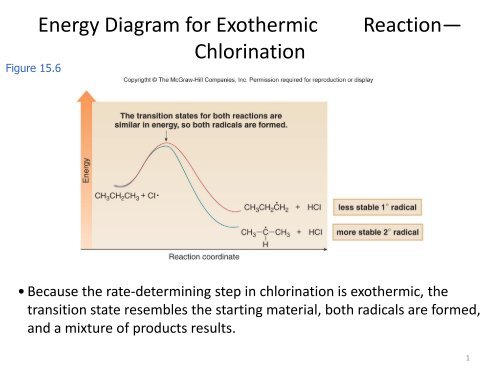
Diagram of exothermic reaction
100% (1 rating) Energy diagram that corresponds to one step exothermic reaction with highest activation energy : Exothermic reaction is exergonic process where energy is released. The initial line of the energy diagram indicates reactants and heap is the process of …. View the full answer. Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2)
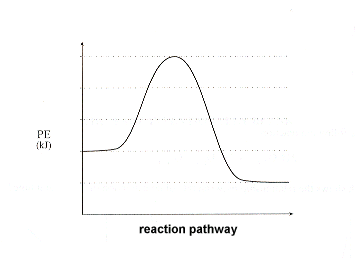
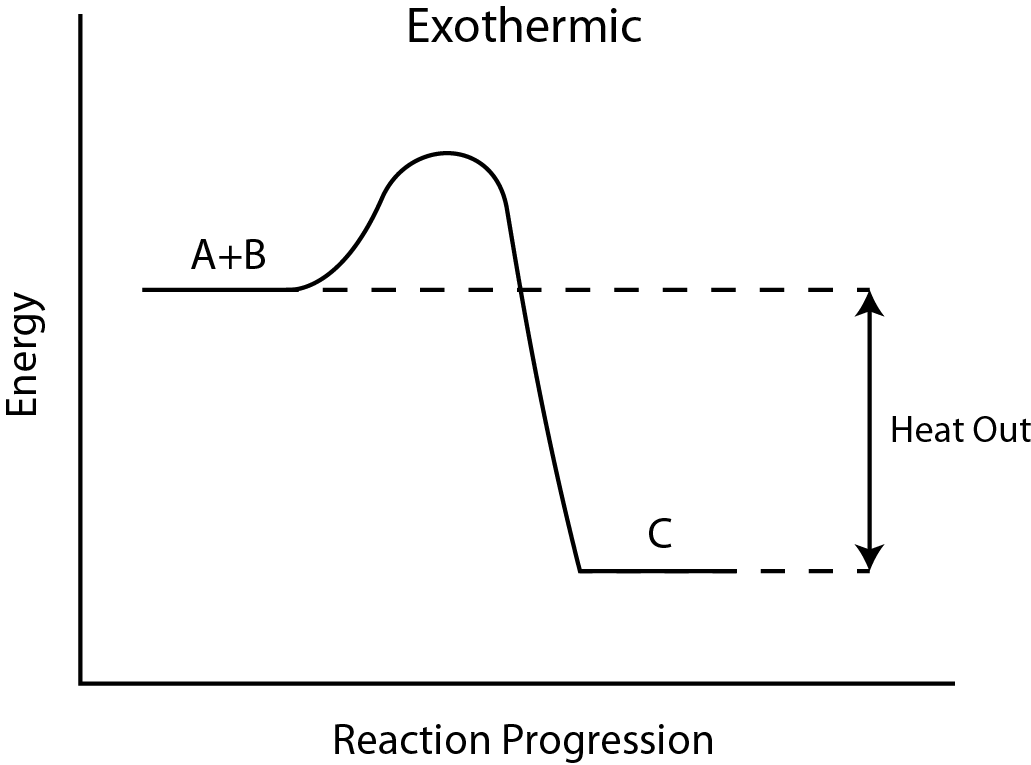
Diagram of exothermic reaction. Exothermic vs. Endothermic and K. An exothermic reaction occurs when the temperature of a system increases due to the evolution of heat. This heat is released into the surroundings, resulting in an overall negative quantity for the heat of reaction ( q r x n < 0 ). An endothermic reaction occurs when the temperature of an isolated system ... An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative. In this example, B is at a lower total energy than A. This is an exothermic reaction (heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram. However some energy is required for A to convert to B. Exothermic Reaction In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products.
Endothermic and Exothermic Reactions. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Endothermic reactions require energy, so energy is a reactant. Heat flows from the surroundings to the system (reaction mixture) and the enthalpy of the system increases (ΔH is positive). Oct 24, 2021 · The energy profile diagram of an exothermic reaction is given below. Diagram of exothermic reaction. The green arrow is longer. Energy Diagram for Exothermic Reaction. Thus in an exothermic reaction energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2) Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
100% (1 rating) Energy diagram that corresponds to one step exothermic reaction with highest activation energy : Exothermic reaction is exergonic process where energy is released. The initial line of the energy diagram indicates reactants and heap is the process of …. View the full answer.





















0 Response to "37 diagram of exothermic reaction"
Post a Comment