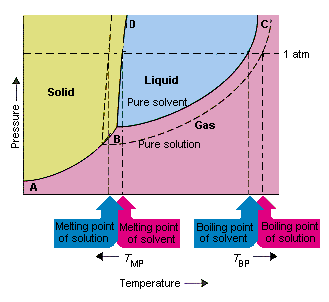
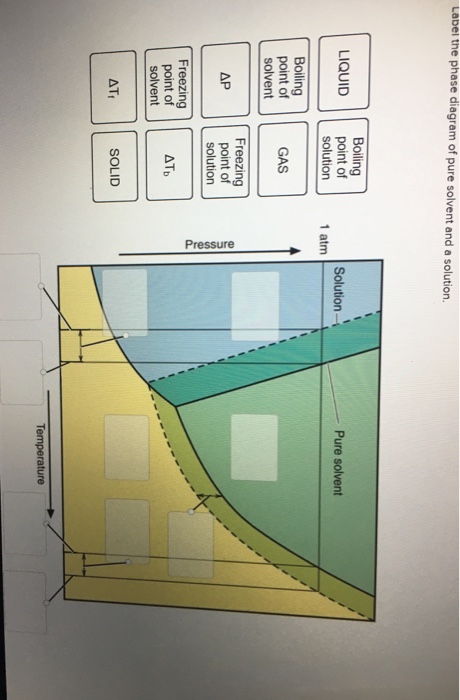
35 label the phase diagram of pure solvent and a solution.
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. This is …. View the full answer. Transcribed image text: Label the phase diagram of pure solvent and a solution. The following can be found from phase diagram if alloy composition and temperature. Label the phase diagram of pure solvent and a solution. The diagram also includes the melting and boiling points of the pure water from the original phase diagram for pure water black lines. A volatile substance will readily. This is the easy one.
- Solutions - solid solutions, single phase - Mixtures - more than one phase • Solubility Limit : Max concentration for which only a single phase solution occurs. Question: What is the solubility limit at 20°C? Answer: 65 wt% sugar . If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure ...

Label the phase diagram of pure solvent and a solution.
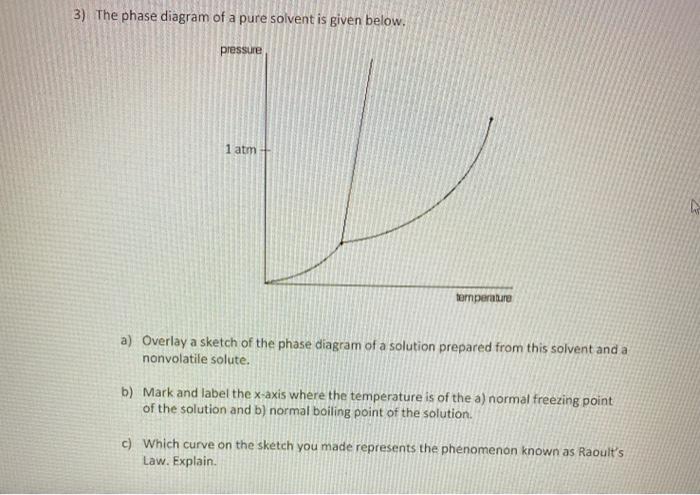
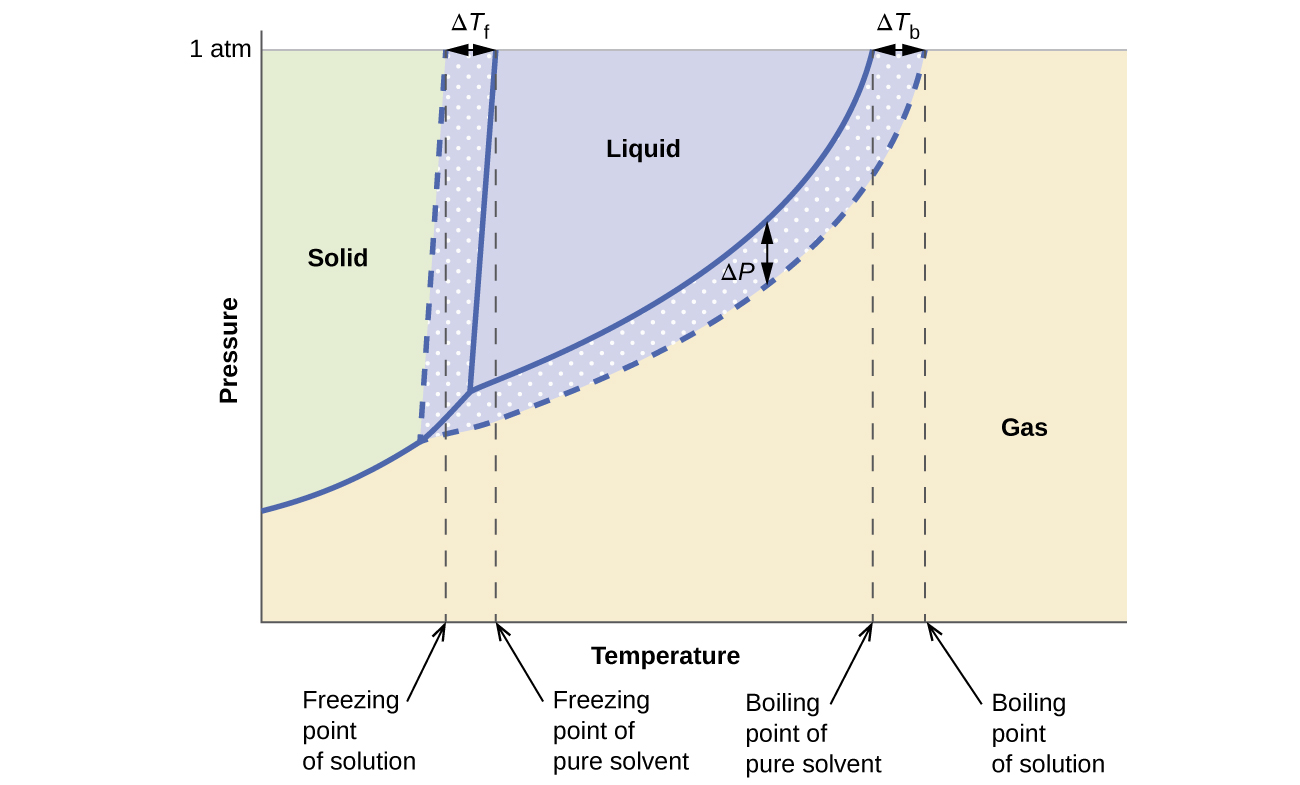
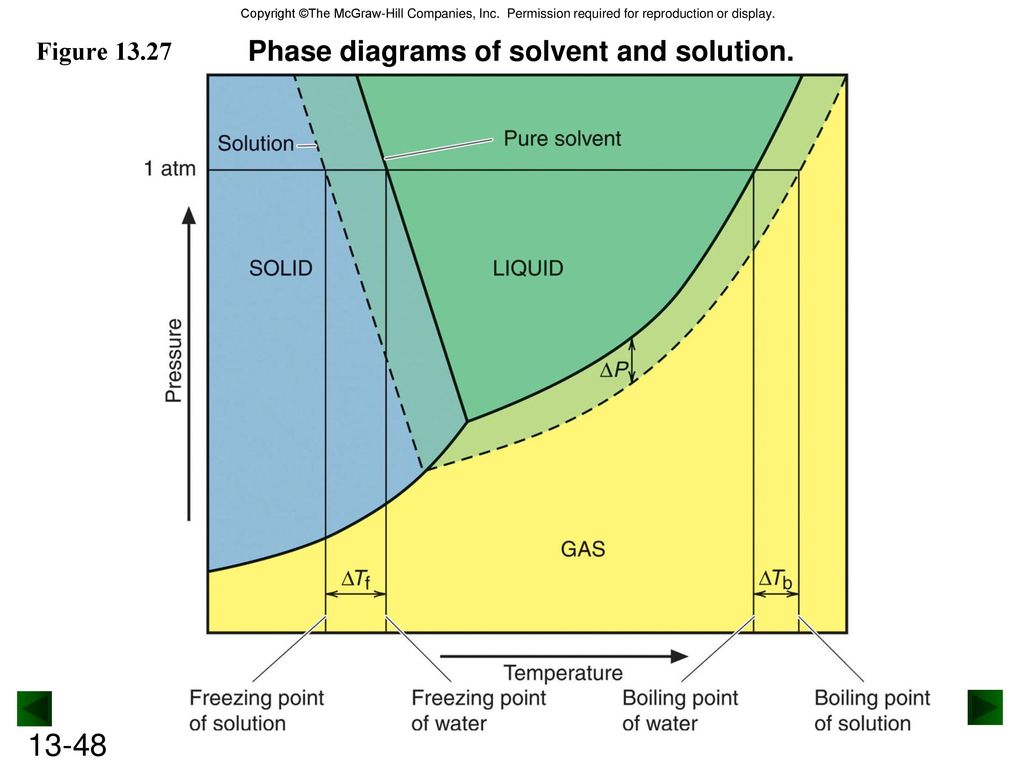
The completely solidified alloy in the phase diagram shown is a solid solution because: Alloying element (Cu, solute) is completely dissolved in host metal (Ni, solvent) Each grain has same composition Atomic radius of Cu is 0.128nm & that of Ni is 0.125nm, Both elements are FCC; HRRs are obeyed. 12/3/2013 11:12 PM Phase Diagram 1. Chapter-5 PHASE AND PHASE EQUILIBRIUM Prepared By: PALLAV RADIA Asst prof. AITS, RAJKOT. 2. Introduction: One of the most important objective of engineering metallurgy is to determine properties of material. The properties of material is a function of the microstructure which depend on the overall composition and variable such as pressure and temperature. Hence to determine ... Transcribed image text: The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fp_solv) and boiling (bp_solv) points for the pure solvent and the normal freezing (fp_soln) and boiling (bp_soln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent.
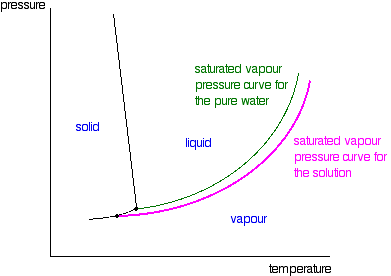
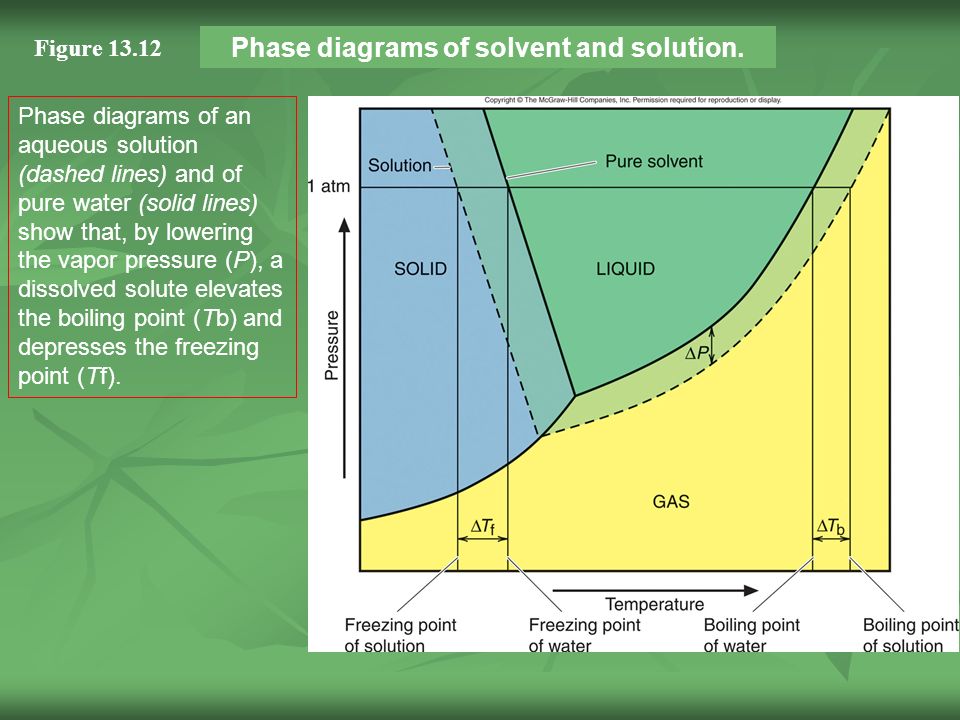
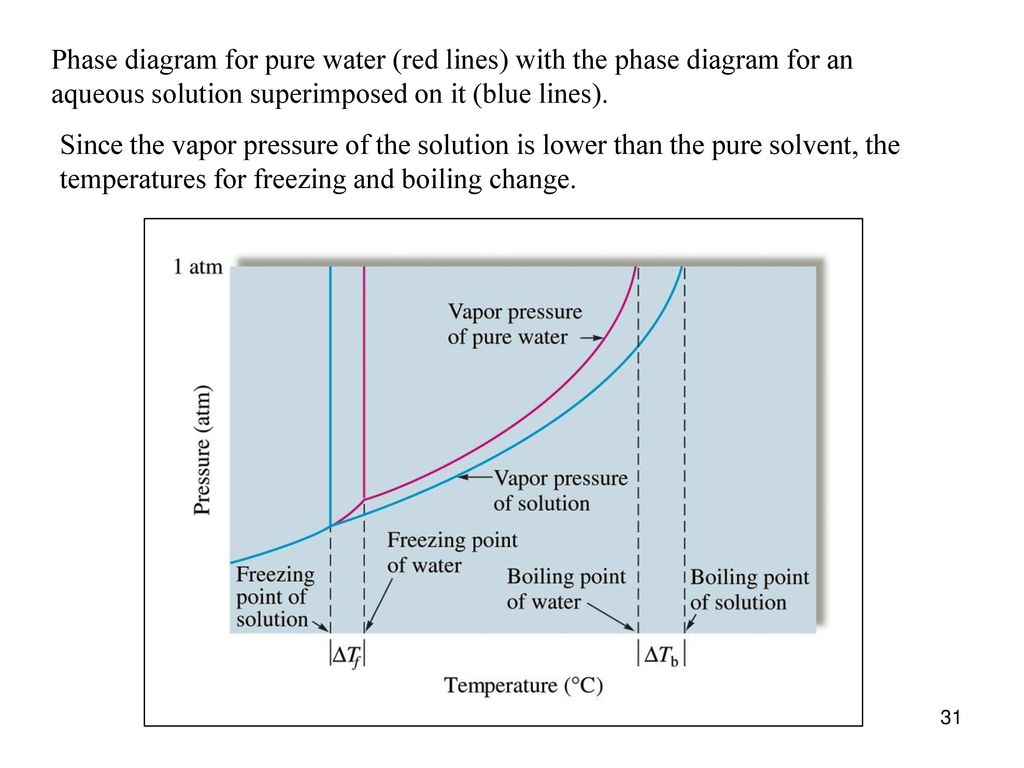
Label the phase diagram of pure solvent and a solution.. Feb 16, 2021 · The diagram also includes the melting and boiling points of the pure water from the original phase diagram for pure water (black lines). Figure \(\PageIndex{8}\): Because of the changes to the phase diagram, you can see that: the boiling point of the solvent in a solution is higher than that of the pure solvent; A phase diagram is a graph which shows under what conditions of temperature and pressure distinct phases of matter occur. The simplest phase diagrams are of pure substances. These diagrams plot pressure on the y-axis and temperature on the x-axis. Although phases are conceptually simple, they are difficult to define precisely. Binary Solutions Solubility Phase Matter. Assignment 3 Winter 2017 With Solutions Studocu. Fragrance Systems Studied In This Work Compris Ing Binary. Label The Phase Diagram Of Pure Solvent And A Solution. Vapour Liquid Equilibrium Vle An Algorithm Inspired. Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
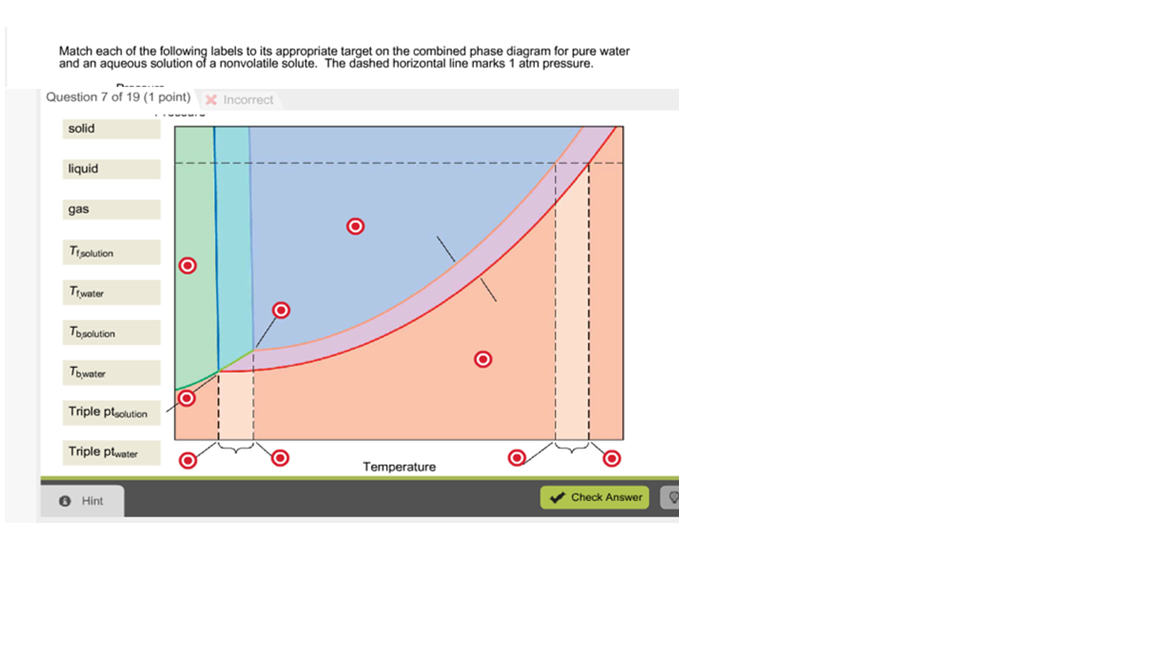
Label the phase diagram of pure solvent and a solution freezing point of solution gas solution pure solvent 1 atm boiling pointfreezing point of of solvent solvent. Phase diagrams are visual tools to display the properties of pure compounds forming various phases at different temperatures and pressures. A volatile substance will readily. Consider the following general phase diagram: Now, consider the pure substance, whose phase diagram was represented by the black curves. The normal freezing point of the pure substance at constant pressure is indicated by B, and its reduced normal freezing point (due to addition of nonvolatile solute to the pure liquid substance) is A, since Tdarr leftwards. Phase Diagrams Revised: 1/27/16 3 The phase diagram in Figure 1 is for a pure compound. When a second compound is introduced to the system forming a homogeneous solution however, the phase diagram drastically changes. For example, the addition of a solute to a pure solvent (making a solution) Label the phase diagram of pure solvent and a solution. Shown above is a phase diagram for water. Well look now at the phase diagram for sodium chloride solution in some detail. Phase diagrams of pure water and an aqueous solution of a nonvolatile solute. Pure a and pure b are also considered to be α and β phases respectively.
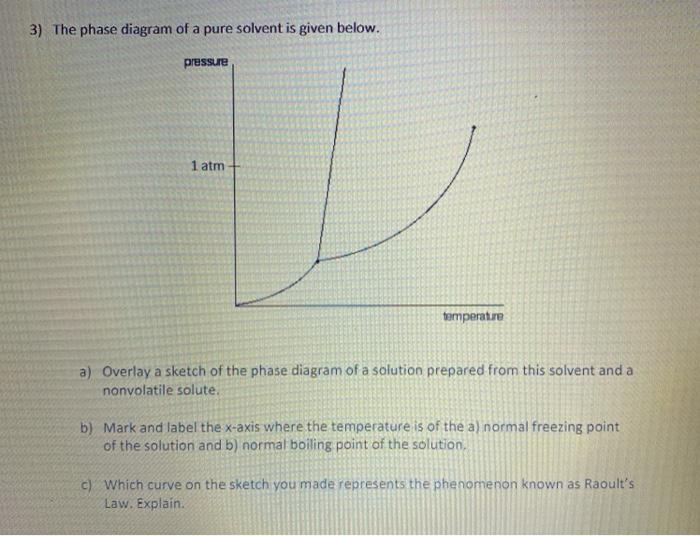
Label the phase diagram of pure solvent and a solution. The vaporization curve for the solution lies below the curve for pure water at all temperatures which results in an increase in the boiling point and a decrease in the freezing point of the solution. This is the case for many solutions comprising liquid solvents and nonvolatile solutes. where the temperature is of the a) normal freezing point Mark and label the x-axis of tne solution and b) normal boiling point of the solution. Raoult'$ Which ... These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation points, boiling points). A typical phase diagram for a pure substance is shown in Figure 1. In phase diagrams these phases are separated from the composition extremes 0 and 100. Assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent. Label the phase diagram of pure solvent and a solution freezing point of solution gas solution pure solvent 1 atm boiling pointfreezing point of of solvent solvent.
B graphite is the most stable phase of carbon at normal conditions. Phase diagrams of pure water and an aqueous solution of a nonvolatile solute ...
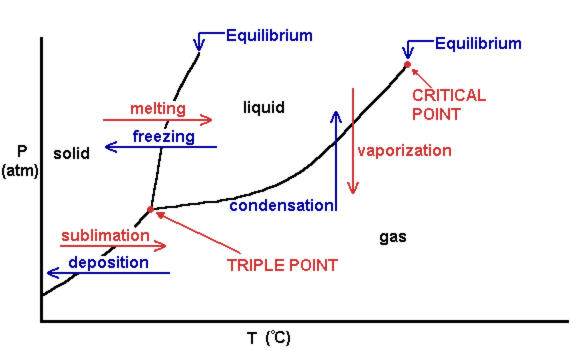
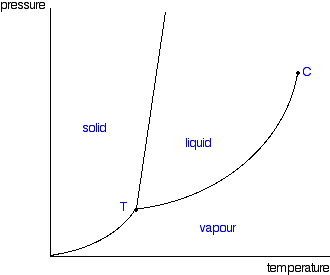
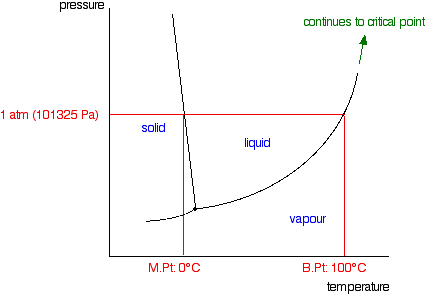
A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2.
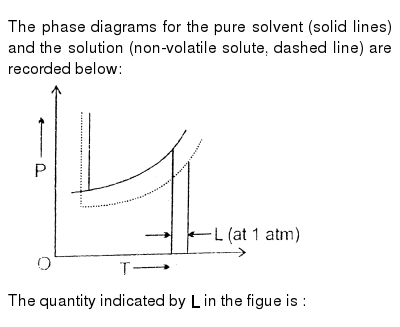
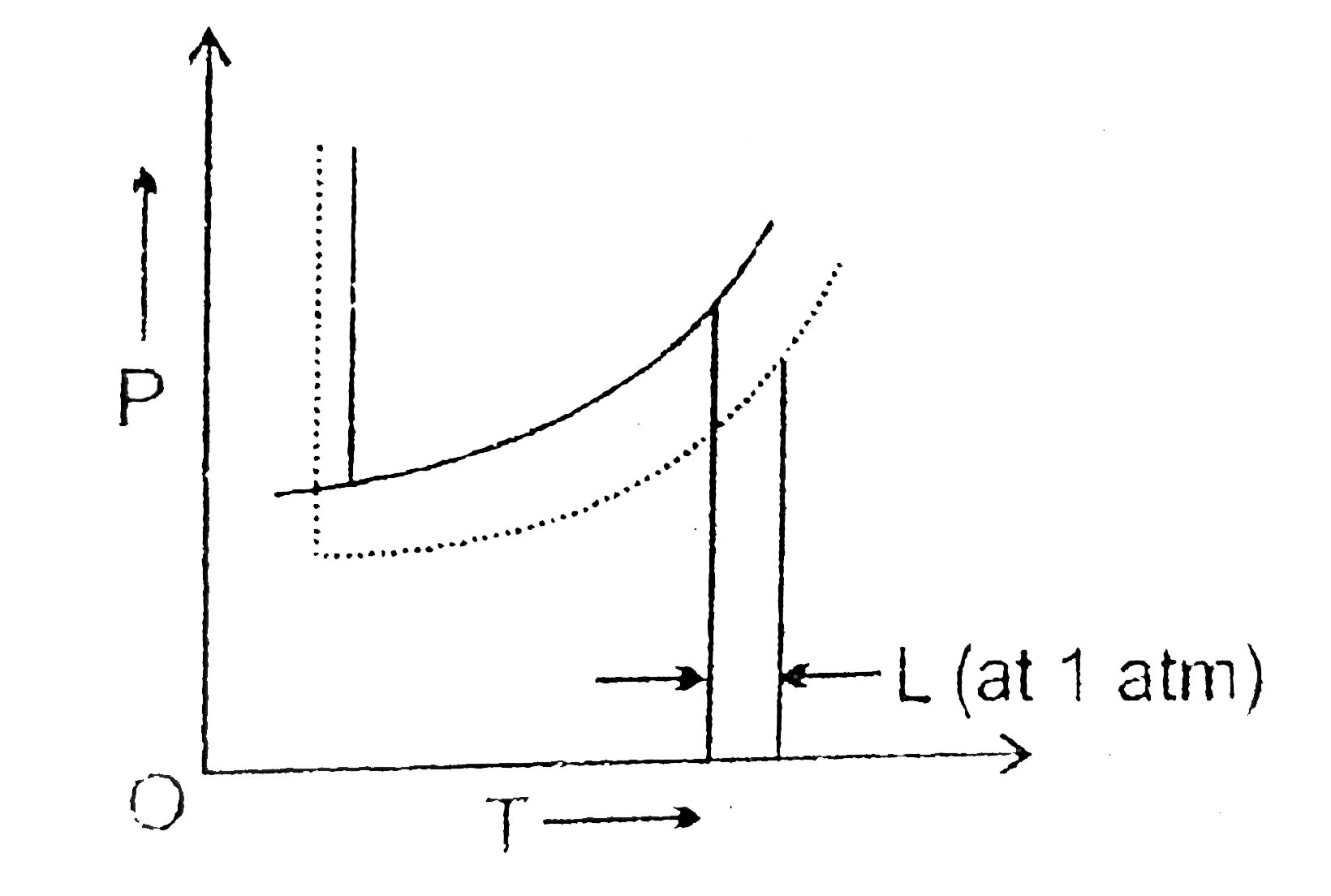
The Phase Diagrams For The Pure Solvent Solid Lines And The Solution Non Volatile Solute Dashed Line Are Recorded Below Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images Res Phy Chm V01 Xii C02 E01 267 Q01 Png Width 80 The
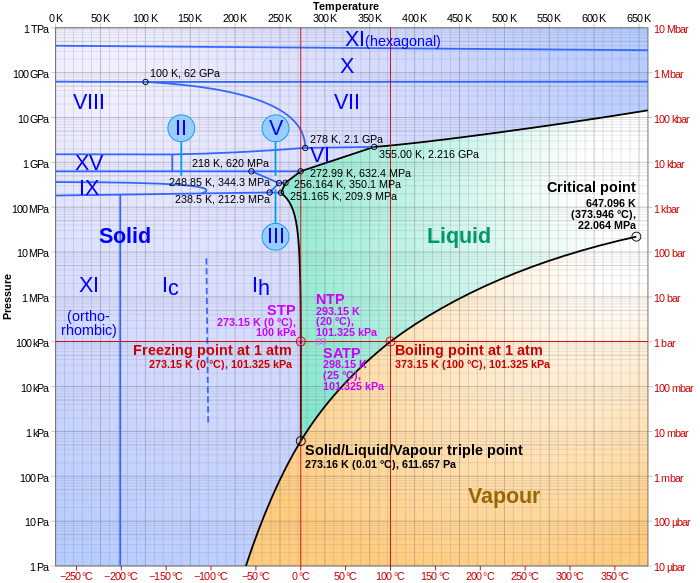
Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.
Chloroform is a colorless, volatile, liquid derivative of trichloromethane with an ether-like odor. Formerly used as an inhaled anesthetic during surgery, the primary use of chloroform today is in industry, where it is used as a solvent and in the production of the refrigerant freon.
Label 3 clean and dry test tubes: •hexane, •70:30 hexane : acetone, and •pure acetone. Name the pigment that we would expect to see near the solvent front and explain why it moves so quickly. Using Paper Chromatography in different solvents to know the Types of Dyes that are used in Food.
the pure solvent is present. Thus the vapor pressure above the solution containing solute will be lower than the vapor pressure of the pure solvent. The dissolved solute molecules physically block the surface of the solvent, preventing some of the solvent molecules from evaporating at a given temperature. Shown below are two phase diagrams for ...
Label the phase diagram of pure solvent and a solution. Which is defined as the maximum amount of solute that will dissolve in a given quantity of solvent at a specific temperature? solubility
HPLC of plasma N-methyl-2-pyrrlidinone indicated a rapid distribution phase followed by a slow elimination phase with a half life of approximately 7 hours for the (14)C and 10 hours for the tritium isotope. Urinary excretion accounted for approx 70% of the total dose within 12 hours, and a 2:1 ratio in the administered dose was maintained in ...
However, the liquid surrounding it, although at approximately 0 C, is a near-saturated salt solution, not pure water. 3 - Page 28 - Is Matter Around Us Pure - Science Class 9th 1A 12. In this laboratory, you will observe the effect of the presence of a common ion on the molar solubility and K sp Solubility Curve Lab And Answerswater and the ...
Problem: Label the diagram of pure solvent and a solution. FREE Expert Solution Recall that a phase diagram shows the transition of matter between solid, liquid, and gas phases as temperature and pressure changes.
Label the phase diagram of a pure solvent and a solution. Answer. +20. Watch. 1. answer. 0. watching. 105. views.
The Figure Shows Two Phase Diagrams One For A Pure Liquid Black Line And The Other For A Solution Made Using The Liquid As The Solvent Red Line What Does Point B
Expt. 5: Binary Phase Diagram CHEM 366 V-3 Combination1,2,3 of equations (2), (3), (6), (7) and (10) leads to the equation T≅TA+ RTA 2 ΔHA lnXA (11) or T≅TA+ RTA 2 ΔHA ln(1−XB) (12) or T≅TA- RTA 2 ΔHA lnXB (13a) T≅TB- RTB 2 ΔHB lnXA (13b) where T and TA are freezing points (K) of the mixture II and pure A, R is 8.314 J/K mole, ΔHA is the molar enthalpy of fusion of A and XB ...
D the average intermolecular distance is decreasing. Label the phase diagram of pure solvent and a solution. Otherwise in β phase solid solution a is the solute. This problem has been solved. Three single phase regions are found on the diagram. Pure a and pure b are also considered to be α and β phases respectively.
III.1. Draw phase diagrams for the following type systems. Label the regions and in-tersections of the diagrams, stating what materials (possibly compounds or azeotropes) are present and whether they are solid, liquid or gas. (a) One component, pressure-temperature diagram, liquid density greater than that of solid. (b) Two component,
(ii) Explain why pure silver chloride could NOT be made by adding silver carbonate to hydrochloric acid. 3. Two students made the insoluble salt, lead sulphate, and wrote these notes about the experiment. ‘We took 25 cm 3 of lead nitrate solution and slowly added 25cm3 of acid to it. The mixture turned cloudy white.

The Phase Diagrams For The Pure Solvent Solid Lines And The Solution Non Volatile Solute Dashed Line Are Recorded Below The Quantity Indicated By L In The Figure Is Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images
The phase diagram for sodium chloride solution. What the phase diagram looks like. ... because adding a non-volatile solute to a solvent increases its boiling point. ... in this case, that's the pure ice crystals. On the other end, it hits the sloping line - this tells you the composition of the remaining salt solution. ...
At 22.0°C the two solutions in equilibrium have x = 0.24 and x = 0.48, respectively, and at 21.5°C the mole fractions are 0.22 and 0.51. Sketch the phase diagram. Describe the phase changes that occur when perfluorohexane is added to a fixed amount of hexane at (a) 23°C, (b) 22°C.
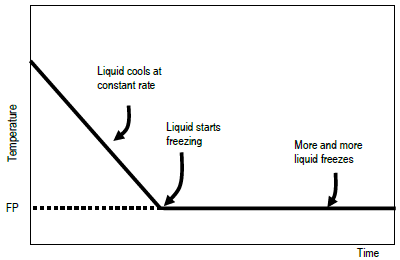
The phase diagram for a pure substance is shown above. Use this diagram and your knowledge about changes of ... Label the axes and label the regions in which the solid, ... Pure Solvent Solution 0 Time 0 5 10 15 20 0 Time 0 5 10 15 20 (b) Information from these graphs may be used to determine the molar mass of the unknown solid.
Label the phase diagram of pure solvent and a solution. Assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent. 12716 3 the phase diagram in figure 1 is for a pure compound. The liquid vapor pressure curve is shifted downward to lower pressures but the solid vapor pressure sublimation curve is unaffected.
Skilled solution 100 25 rankings this downside has been solved. Label the phase diagram of pure solvent and a solution. At that point the substance exists as a mixture of gas liquid and solid all in equilibrium with one another. 11 solid at ap boiling pointliquid of solution temperature. Mole fraction of the pure solvent vp of the pure solvent.
Freezing point of solution is the dotted line temperature as we know that there is a depression is the freezing point of solution. The pure solvent freezing ...
Transcribed image text: The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fp_solv) and boiling (bp_solv) points for the pure solvent and the normal freezing (fp_soln) and boiling (bp_soln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent.
Phase Diagram 1. Chapter-5 PHASE AND PHASE EQUILIBRIUM Prepared By: PALLAV RADIA Asst prof. AITS, RAJKOT. 2. Introduction: One of the most important objective of engineering metallurgy is to determine properties of material. The properties of material is a function of the microstructure which depend on the overall composition and variable such as pressure and temperature. Hence to determine ...
The completely solidified alloy in the phase diagram shown is a solid solution because: Alloying element (Cu, solute) is completely dissolved in host metal (Ni, solvent) Each grain has same composition Atomic radius of Cu is 0.128nm & that of Ni is 0.125nm, Both elements are FCC; HRRs are obeyed. 12/3/2013 11:12 PM

Solid Liquid Phase Equilibria Of The Ternary System Nacl Ch3oh H2o At 298 15 308 15 318 15 K And 0 1 Mpa









0 Response to "35 label the phase diagram of pure solvent and a solution."
Post a Comment