35 in this phase diagram for water indicate the direction that the solid-liquid and liquid-gas
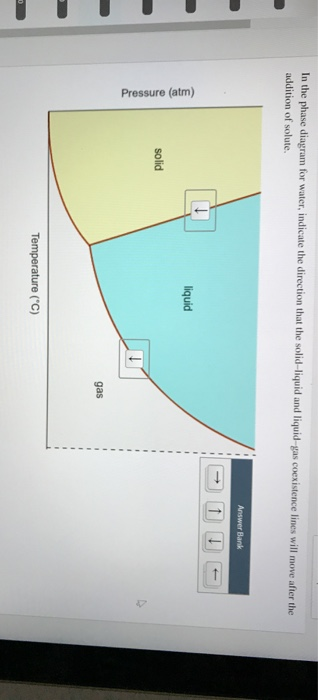
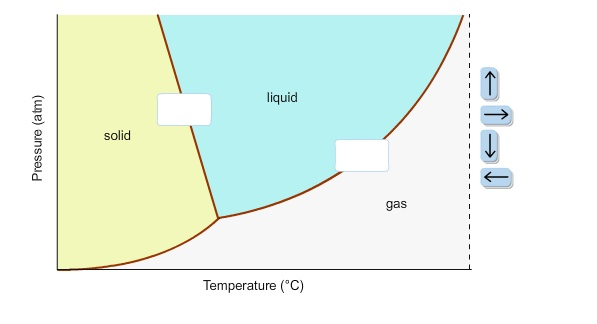
In this phase diagram for water indicate the direction that the solid liquid and liquid gas. In this phase diagram for water indicate the direction that the solid liquid and liquid gas coexistence lines will move after the addition. Lines on a phase diagram represent a point where the two phases on either side of the line are in equilibrium. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases.
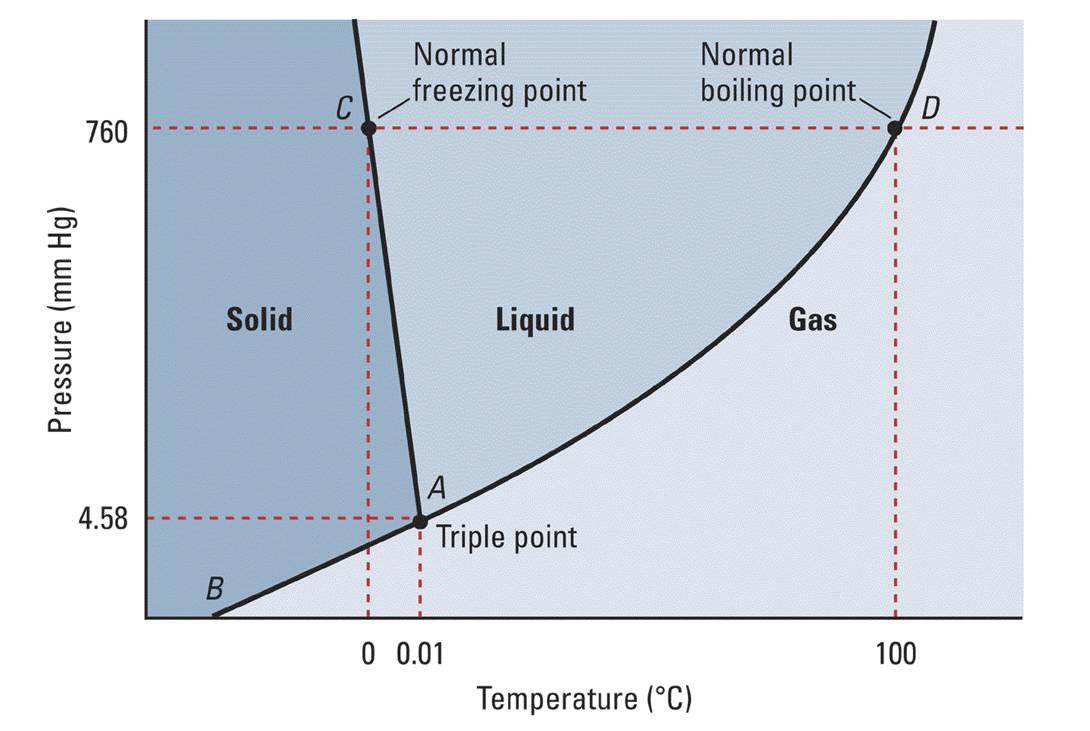
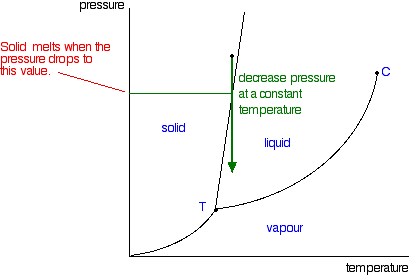
The phase diagram explains why liquids do not exist in space or any other vacuum. Since the pressure is zero (except on surfaces or interiors of planets and moons) water and other liquids exposed to space will either immediately boil or freeze depending on the temperature. In regions of space near the earth, water will freeze if the sun is not shining directly on it and vaporize (sublime) as ...

In this phase diagram for water indicate the direction that the solid-liquid and liquid-gas
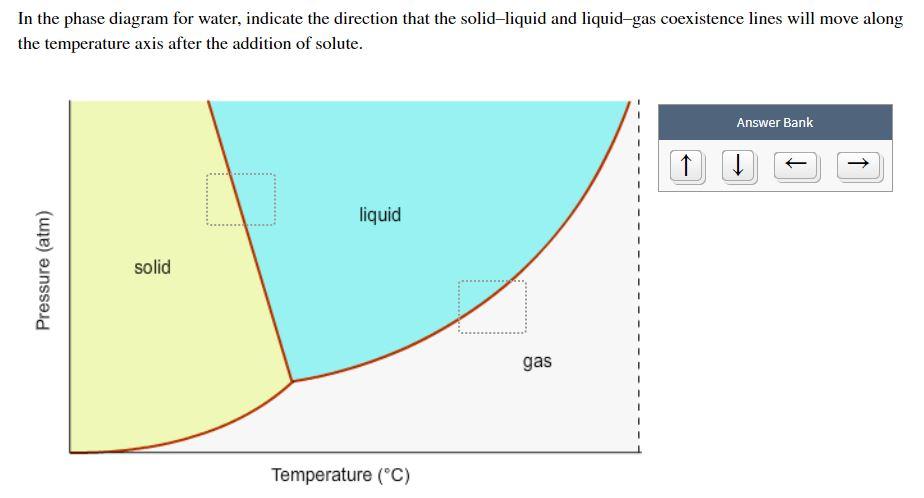
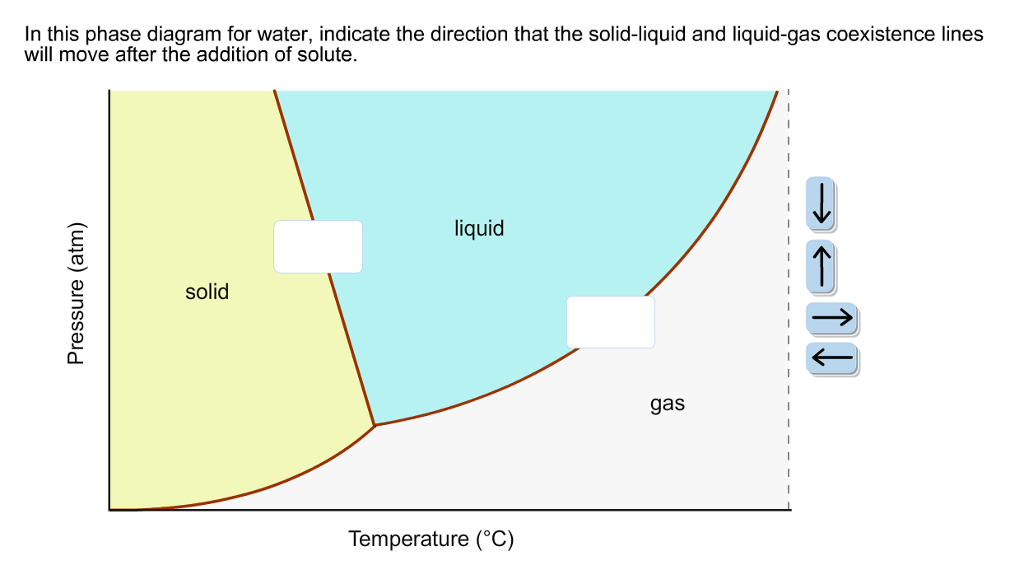
Problem. : In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. FREE Expert Solution. After the addition of solute, the solid - liquid coexistence line will move towards lower temperatures. 95% (314 ratings) In this diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Solid <----- Liquid Liquid -----> Gas. 10. A solution is made by dissolving 0.617 mol of nonelectrolytes solute in 889 g of benzene. Calculate the freezing point, Tf, and boiling point Tb of the solution. In chemistry, thermodynamics, and many other related fields, phase transitions (or phase changes) are the physical processes of transition between a state of a medium, identified by some parameters, and another one, with different values of the parameters. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, as well as plasma in rare cases.
In this phase diagram for water indicate the direction that the solid-liquid and liquid-gas. 25.10.2021 · "contaminant" means any solid, liquid, gas, odour, heat, sound, vibration, radiation or combination of any of them resulting directly or indirectly from human activities that causes or may cause an adverse effect. For RSCs, the standards for selected contaminants are set out in the "Soil, Ground Water and Sediment Standards for Use under Part XV.1 of the Environmental Protection Act". These ... In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Calculate the osmotic pressure of a 0.186 M aqueous solution of sucrose, C12H22O11, at 37*C. In this phase diagram for water indicate the direction that the solid liquid and liquid gas coexistence lines will move after the addition of solute. This chemistry video tutorial explains the concepts behind the phase diagram of co2 carbon dioxide and the phase diagram of water h2o. A semipermeable membrane separates two aqueous solutions at 20 c. Transcribed image text: In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the ...
The water molecules are actually closer together in the liquid phase than they are in the solid phase. Refer again to water’s phase diagram ( Figure above ). Notice point E, labeled the critical point . What does that mean? At 373.99°C, particles of water in the gas phase are moving very, very rapidly. At any temperature higher than that ... Transcribed image text: In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the ... In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. 15. Menthol is a crystalline substance with a peppermint taste and odor.
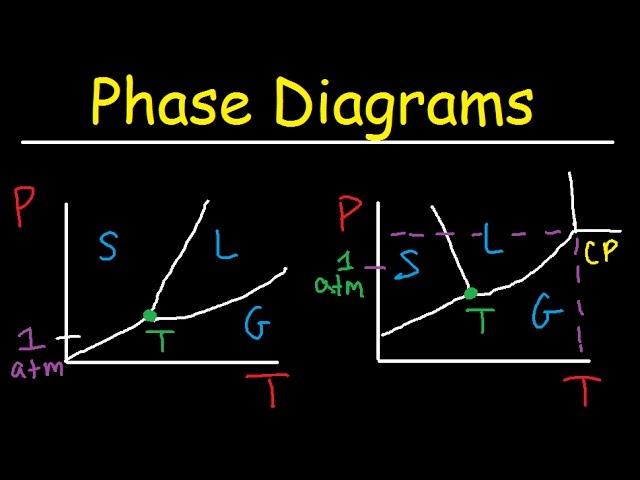
In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Show transcribed image text In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Transcribed image text: In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the ... In the phase diagram of water, the solid-liquid coexistence line will move towards left after the addition of solute because of depression in freezing point of ...1 answer · Top answer: Concepts and reason The concept used to solve this problem is the phase diagram of water. In the phase diagram of water, the solid-liquid coexistence ... Phase Diagram for a Substance: The figure highlights the critical point, above which (in either temperature or pressure) the substance does not exist in either the liquid or gas phase. Under those conditions it is called a “supercritical fluid,” and has properties between those of a liquid and a gas. It can effuse through solids (like a gas), and dissolve materials (like a liquid). In ...
The curve is called a solidus, liquidus, or vaporus depending on whether phase \(\pha\) is a solid, liquid, or gas. A binary system with three phases has only one degree of freedom and cannot be represented by an area on a two-dimensional phase diagram. Instead, there is a horizontal boundary line between areas, with a special point along the line at the junction of several areas. The ...
Transcribed image text: In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the ...
Question: In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis ...
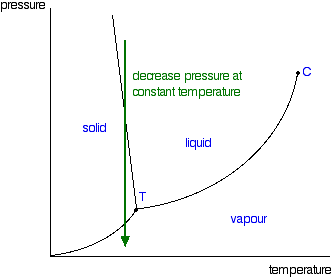
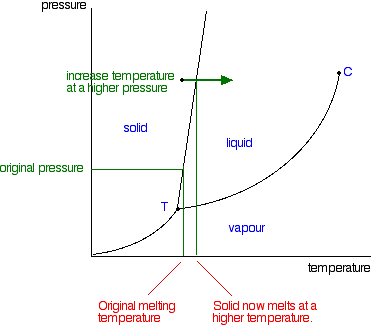
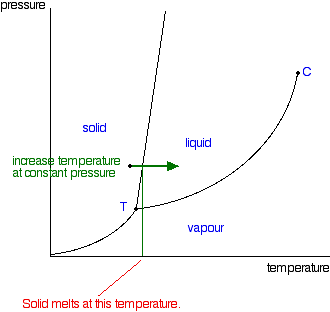
delineates the boundaries of the phase fields, it does not indicate the quantity of any phase present. It is of interest to consider the slope of the liquid/solid phase line of the H. 2O phase diagram. It can readily be seen that if ice – say at –2°C – is subjected to high pressures, it will transform to liquid H2O. (An ice skater will skate not on ice, but on water.) This particular ...
This diagram shows the major steps in the refrigeration cycle. For a description of each step (indicated by the green numbers), see the numbered steps below. In this figure, blue dots represent Freon gas, and solid blue areas represent liquid Freon. Small arrows indicate the direction of heat flow into or out of the refrigerator coils.
In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Answer Bank 1. → liquid Pressure (atm) solid gas Temperature (°C) Question: In the phase diagram for water, indicate the direction that the solid-liquid and liquid ...
Transcribed image text: In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the ...
03.05.2021 · Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). However for other substances, notably water, the line slopes to the left as the diagram for water shows. This indicates that the liquid phase is more dense than the solid phase. This phenomenon is caused by the crystal structure of the solid phase. In the solid forms of water and ...
Solid Liquid Phase Diagrams Salt Solution For each of the following cases name the solution into which a net flow of water if any will occur. In this phase diagram for water indicate the direction that the solid liquid and liquid gas. At 2 it would be a liquid. When moving between solid to gas phases the material undergoes sublimation.

In This Phase Diagram For Water Indicate The Direction That The Solid Liquid And Liquid Gas Wiring Site Resource
In chemistry, thermodynamics, and many other related fields, phase transitions (or phase changes) are the physical processes of transition between a state of a medium, identified by some parameters, and another one, with different values of the parameters. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, as well as plasma in rare cases.
In this diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Solid <----- Liquid Liquid -----> Gas. 10. A solution is made by dissolving 0.617 mol of nonelectrolytes solute in 889 g of benzene. Calculate the freezing point, Tf, and boiling point Tb of the solution.
Problem. : In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. FREE Expert Solution. After the addition of solute, the solid - liquid coexistence line will move towards lower temperatures. 95% (314 ratings)
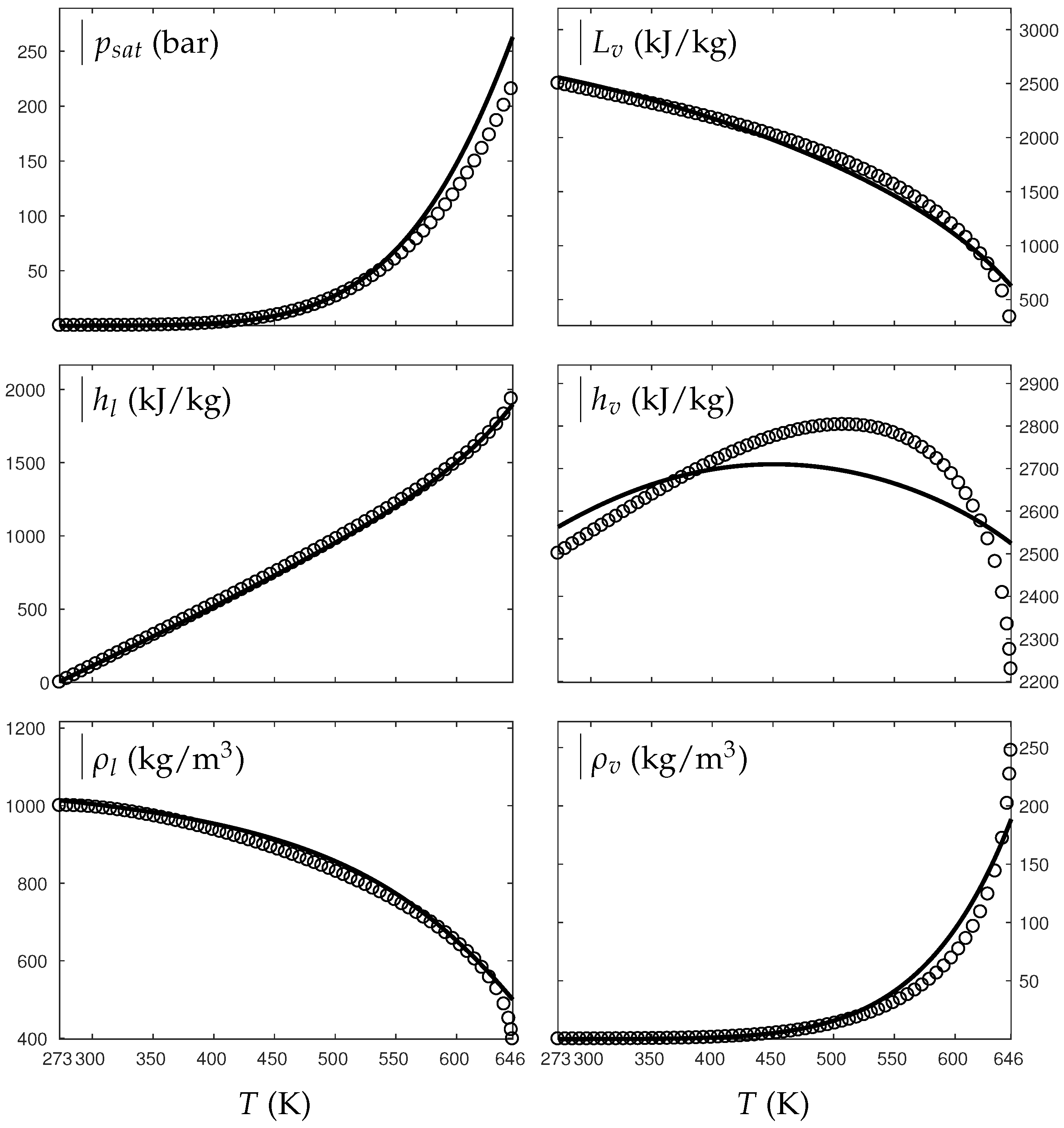
Fluids Free Full Text Extended Noble Abel Stiffened Gas Equation Of State For Sub And Supercritical Liquid Gas Systems Far From The Critical Point Html
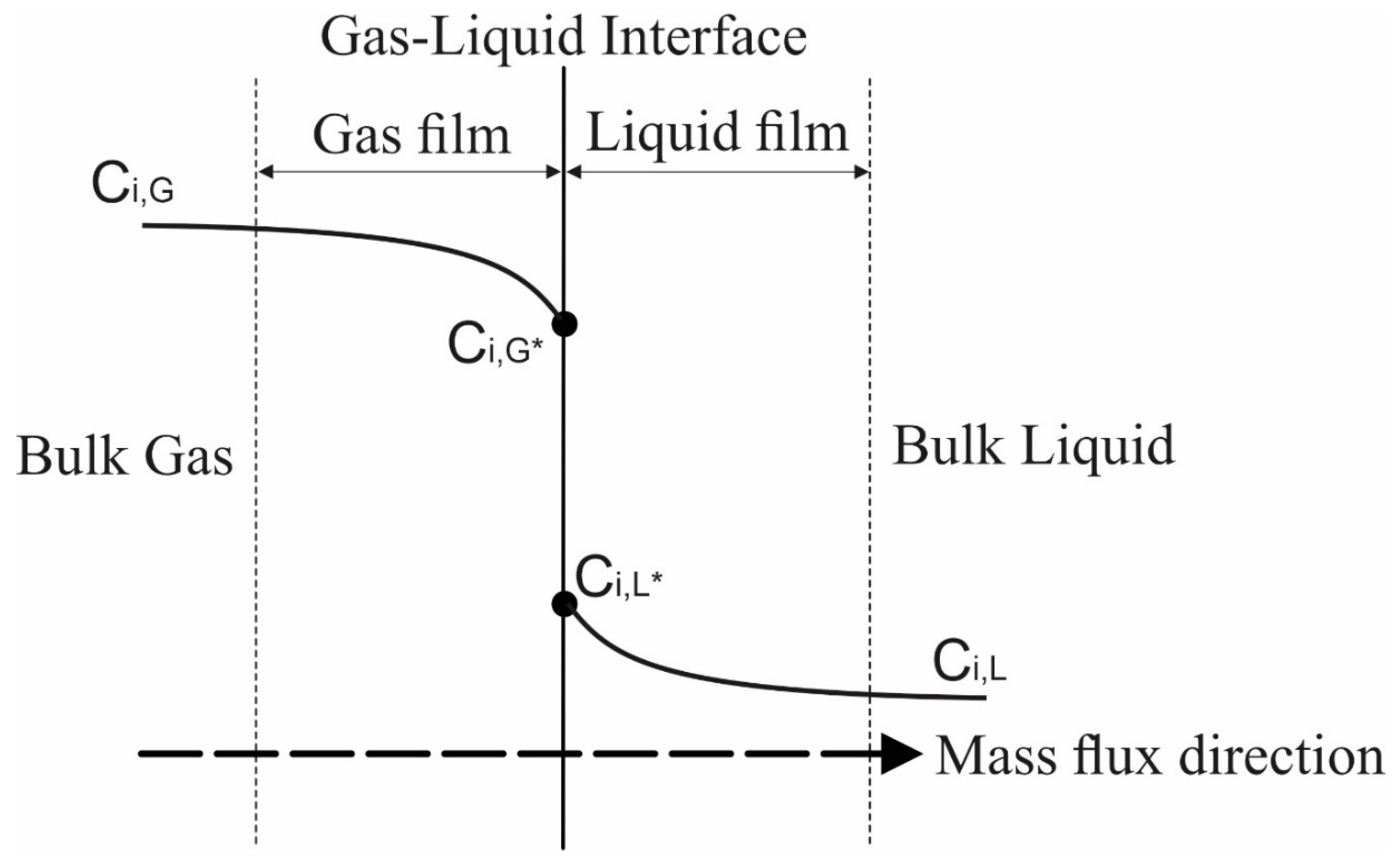
Applied Sciences Free Full Text Influence Of Pressure On Gas Liquid Interfacial Area In A Tray Column Html
In This Phase Diagram For Water Indicate The Direction That The Solid Liquid And Liquid Gas Wiring Site Resource











0 Response to "35 in this phase diagram for water indicate the direction that the solid-liquid and liquid-gas"
Post a Comment