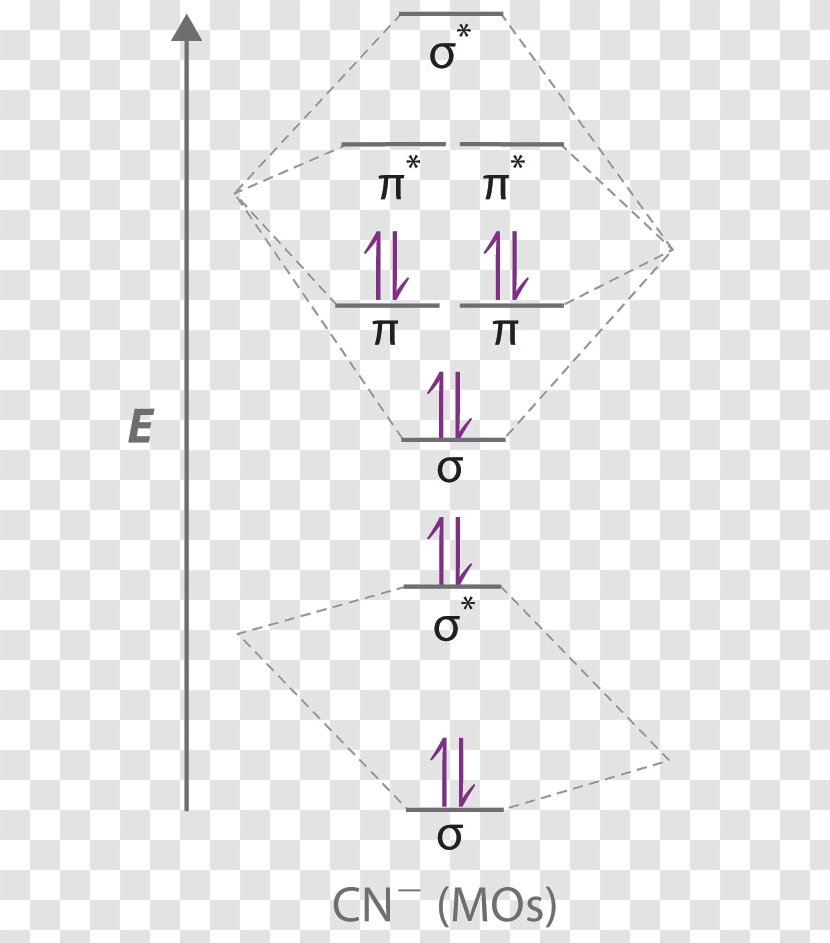
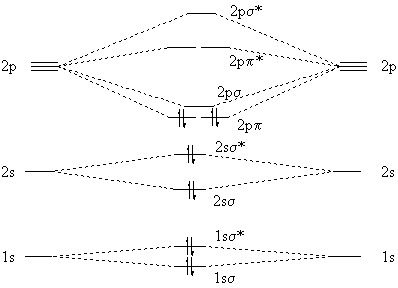
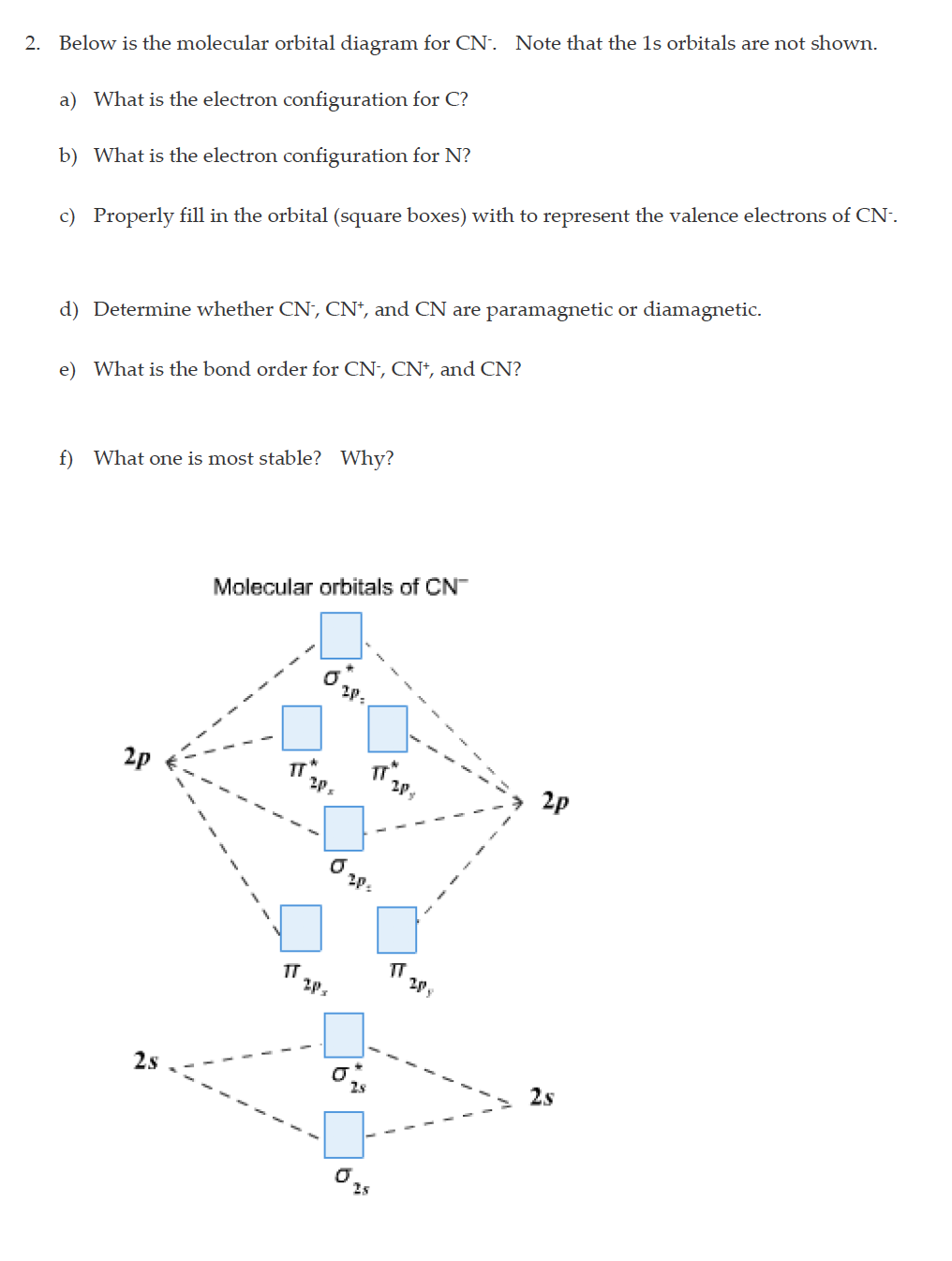
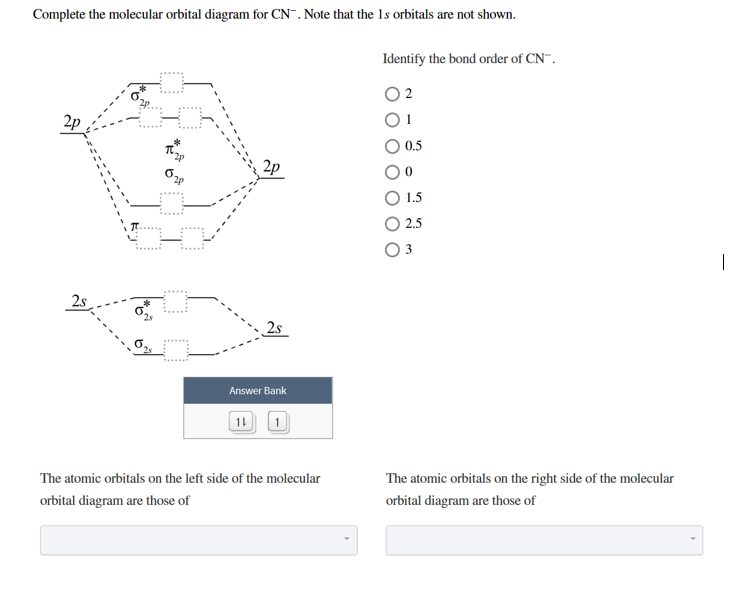
35 complete this molecular orbital diagram for cn
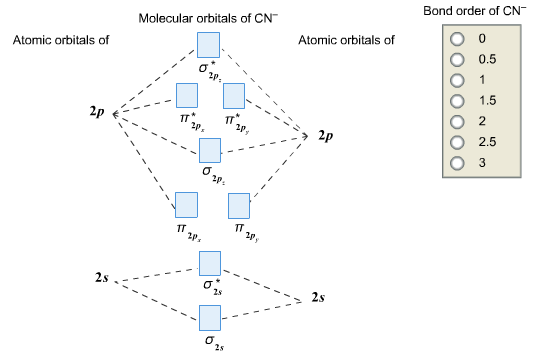
How to make molecular Orbital diagramhttps://www.youtube.com/watch?v=UYC-ndQ6Lww&t=6s Question: Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN. O2 01 OOOOO 25- 0 2s Answer Bank The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of.
21 May 2014 — I could not find the molecular orbital diagram for molecule CN. Can you please describe the MO diagram of CN? Is it the same as 13 electron ...3 answers · 1 vote: Here is the MO for CN-, just take away a single electron from the MO since CN is neutral. Vijayta Gupta is right, the N atom is lower in energy. http://2012book ...

Complete this molecular orbital diagram for cn
Complete this molecular orbital diagram for CN then determine the bond arder. Note that the 1s orbitals not shown in this problem. To add arrows to the MO diagram, dick on the blue boxes. Moleaular orbitals of CN Bond order of CN Atomic orbitals of Select anpwer Atomic orbitals of r B Select answer O 05 O 2 O 25 2s 2s. To add arrows to the MO diagram, click on the blue boxes. Labels. Chemistry. Answers. Bond order of CN- is 2 =No of antibonding ... Complete the molecular orbital diagram for CN−. Note that the 1𝑠 orbitals are not shown. Identify the bond order of CN−. The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of
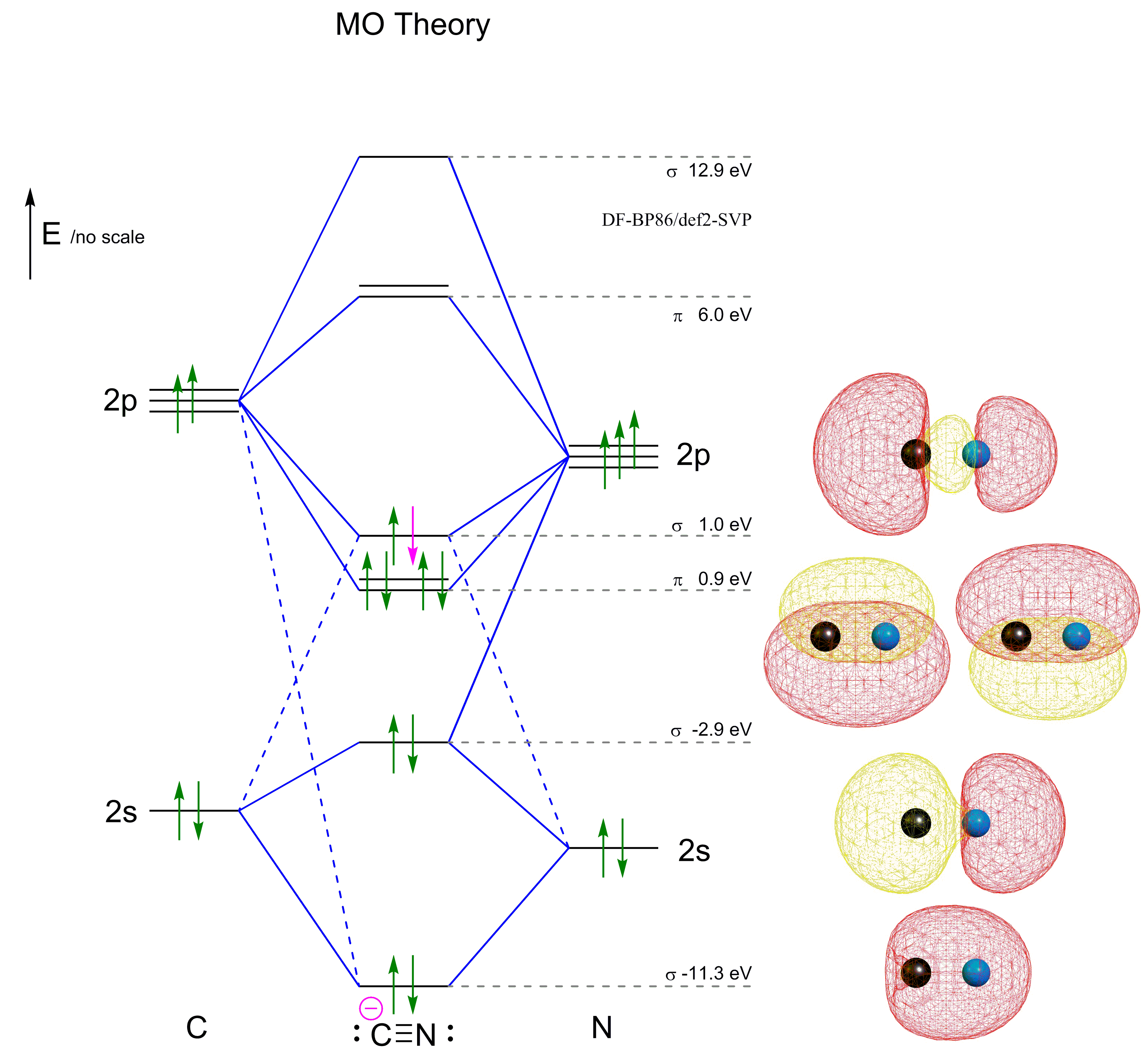
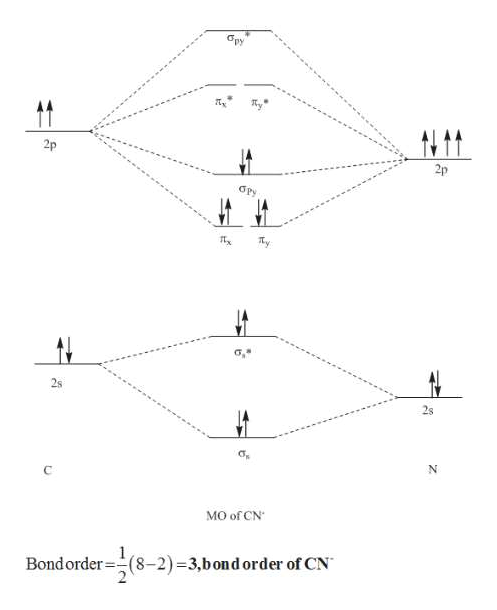
Complete this molecular orbital diagram for cn. 20. Calculate the bond order and determine the magnetism for CN +, CN, and CN-. Which molecule/ion would molecular orbital theory predict to be the most stable? 21. The nitric oxide molecule readily loses one electron to form the NO + ion. (a) Using molecular orbital theory, explain why this observation makes sense chemically. The molecules are said to be stable if the number of electrons in the bonding molecular orbitals is greater than that in anti-bonding molecular orbitals. • Bond ...1 answer · Top answer: Concepts and reason • The molecular orbital theory explains the bonding in terms of the combination and organization of atomic orbitals of an atom which ... FREE Expert Solution. We’re being asked to complete the molecular orbital diagram of CN- and then determine the bond order. To do so, we shall follow these steps: Step 1: Calculate the total valence electrons present. Step 2: Fill the molecular orbitals with electrons. Step 3: Determine the bond order. Step 1: Calculate the total valence ... Answer (1 of 5): \text{Bond order} = \frac{n_{\text{bonding electrons}}-n_{\text{antibonding electrons}}}{2} Here is the molecular orbital diagram of CN-: There are 8 bonding electrons and 2 antibonding electrons, therefore B.O.=\frac{8-2}{2}=3 Here’s a guide on how to construct MO diagrams, i...
MO Theory: Heteronuclear Diatomic Molecules Video Lessons. Concept: Concept: Example: Problem: Complete this molecular orbital diagram for CN– then determine the bond order. Note that the 1s orbital is not shown in this problem. FREE Expert Solution. 83% (108 ratings) play-rounded-fill. Molecular orbital Diagram Cn-mo diagram of cn hunt research group right you have been asked to draw the mo diagram for cn a heteronuclear diatomic don t panic take it one step at a time and you will have a plete mo diagram before you know it this is meant to be an interactive exercise so arrange for some pieces of blank paper a pencil a pen and an eraser molecular orbital theory heteronuclear ... Note that the 1s orbital is not shown in this problem. To add arrows to the MO diagram, click on the blue boxes. Bond order of CN O 0 O 0.5 Molecular orbitals ... Complete the molecular orbital diagram for CN−. Note that the 1𝑠 orbitals are not shown. Identify the bond order of CN−. The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of
To add arrows to the MO diagram, click on the blue boxes. Labels. Chemistry. Answers. Bond order of CN- is 2 =No of antibonding ... Complete this molecular orbital diagram for CN then determine the bond arder. Note that the 1s orbitals not shown in this problem. To add arrows to the MO diagram, dick on the blue boxes. Moleaular orbitals of CN Bond order of CN Atomic orbitals of Select anpwer Atomic orbitals of r B Select answer O 05 O 2 O 25 2s 2s.

Complete This Valence Molecular Orbital Diagram For Oxygen O2 Click The Blue Boxes To Add Electrons As Homeworklib
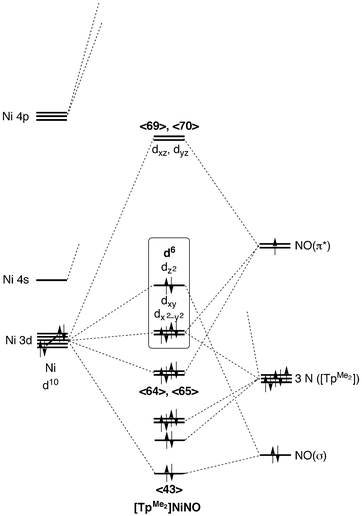
Tetrahedral Nickel Nitrosyl Complexes With Tripodal N 3 And Se 3 Donor Ancillary Ligands Structural And Computational Evidence That A Linear N Dalton Transactions Rsc Publishing Doi 10 1039 B616674a

How Can One Tell From The Mo Diagram Of The Cyanide Ion That The Homo Is Carbon Centred Chemistry Stack Exchange
Solved Compare The Mo Diagram Of Pt Cn 4 2 And Pt Pyridine 4 2 Assume The Pyridine Molecules Are All Flat In The Xy Plane Pyridine Is A Pi Acce Course Hero
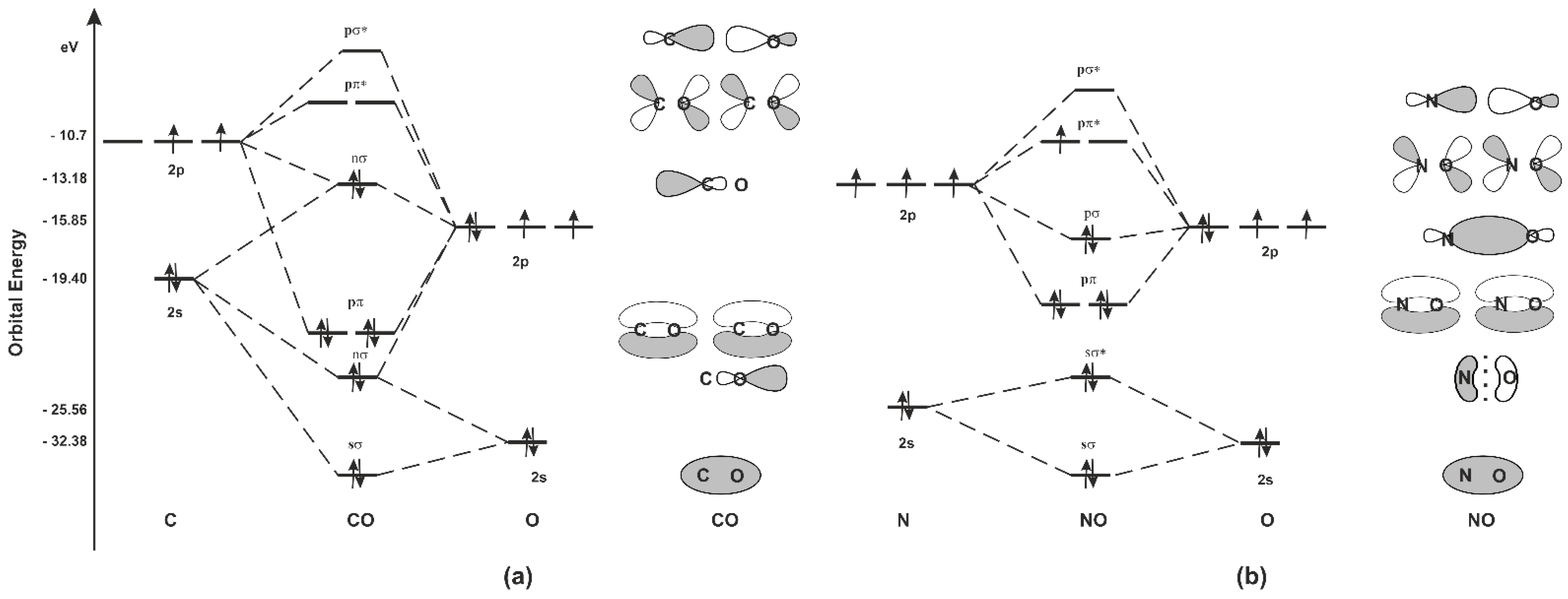
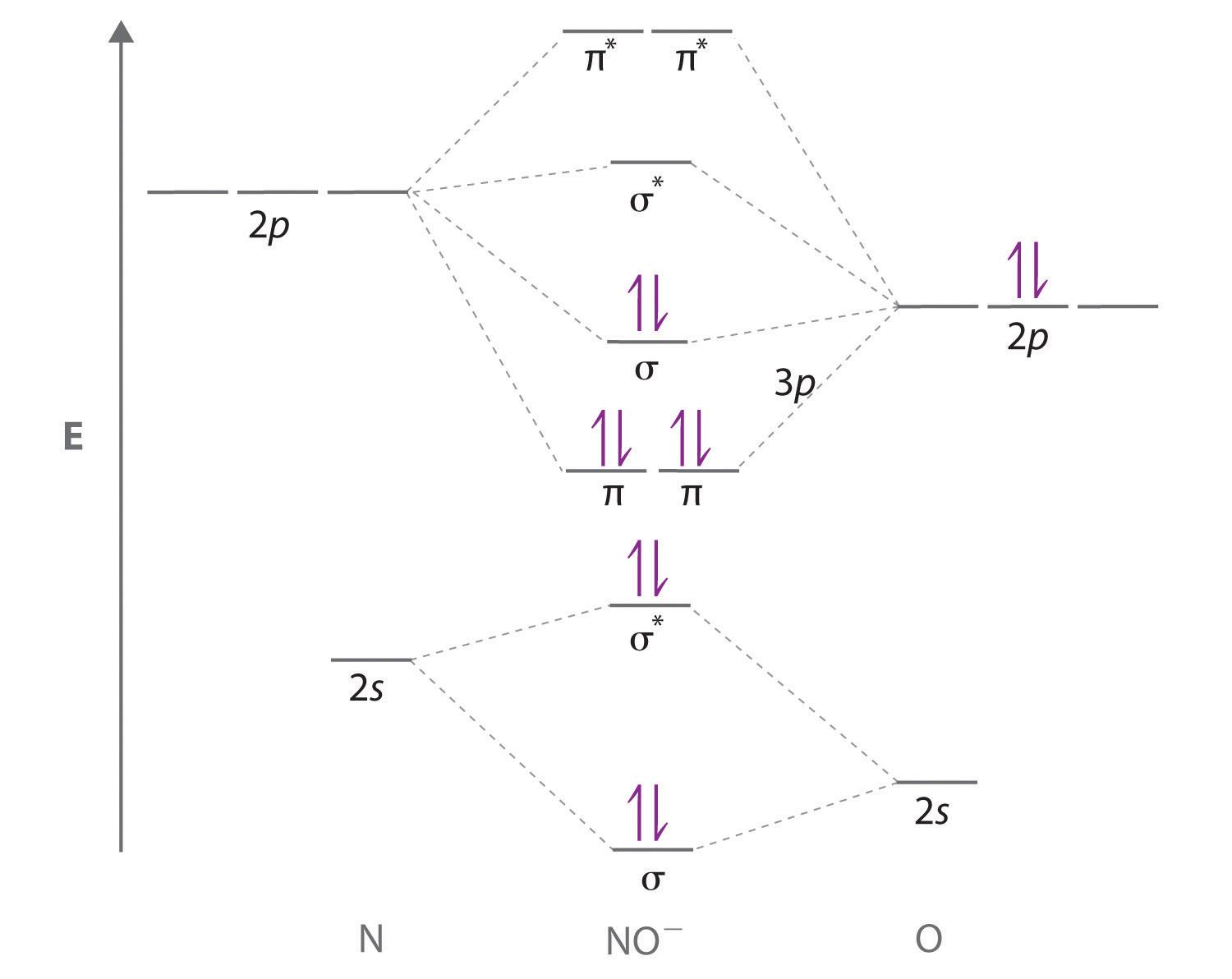
Ijms Free Full Text Carbon Monoxide And Nitric Oxide As Examples Of The Youngest Class Of Transmitters Html

Draw The Molecular Orbital Diagrams For The Following Diatomic Molecules Polyatomic Ions Indicate Their Bond Orders And Rank Them In Order Of Increasing Bond Strength A Cn B Co C F 2 D N 2

Figure 7 From Electronic Structure And Spectroscopy Of Luminescent Heterobimetallic Pt Ii Rh I Au I Rh I And Ag I Rh I Complexes Semantic Scholar
Complete This Molecular Orbital Diagram For Cn Then Determine The Bond Order Note That The 1s Orbit Not Shown In This Problem To Add Arrows To The Mo Diagram Click On The
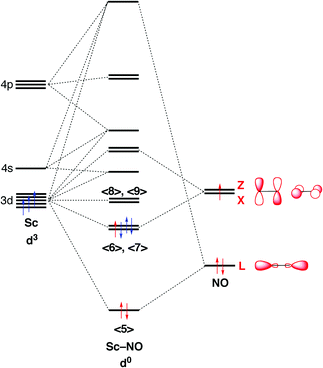
Tetrahedral Nickel Nitrosyl Complexes With Tripodal N 3 And Se 3 Donor Ancillary Ligands Structural And Computational Evidence That A Linear N Dalton Transactions Rsc Publishing Doi 10 1039 B616674a

Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11






0 Response to "35 complete this molecular orbital diagram for cn"
Post a Comment