38 orbital diagram for copper
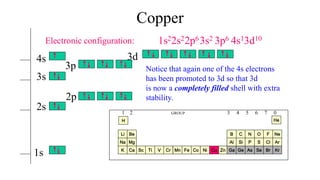
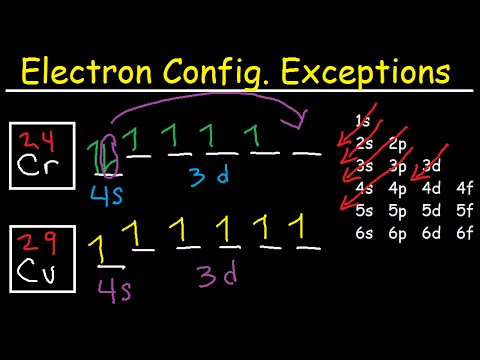
Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table.This would make the electron configuration for copper, #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^9# or in noble gas configuration [Ar] #4s^2 3d^9#.. However, because the 3d orbital is so much larger then the 4s orbital and the 3d orbital only needs one more electron to be filled, the ... Answer to: Draw and explain the orbital diagram for copper (Z = 29). By signing up, you'll get thousands of step-by-step solutions to your homework...
I need to construct the molecular orbital diagram for the hypothetical species Li4, which has the following geometrical arrangement: https://preview.redd.it/npsjre5pch571.png?width=197&format=png&auto=webp&s=c2a7948c2efa04a975bee1db722838fae7482456 The first step is to identify the point symmetry group. In this particular case, we consider that there is only one axis of rotation of order four (actually, other symmetry elements can be observed, but this is a previous consi...

Orbital diagram for copper
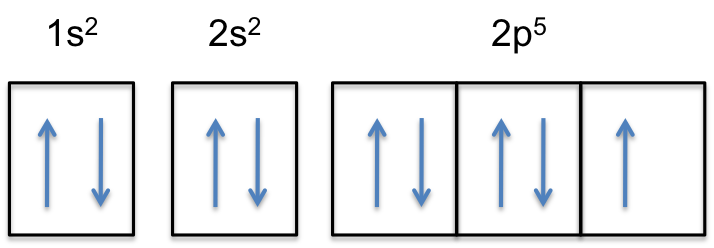
Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the next six electrons. Jun 14, 2012 · The electron configuration of copper is 1s22s22p63s23p63d104s1. Cu (Copper) is an element with position number 29 in the periodic table. Located in the IV period. Melting point: 1083.5 ℃. Density: 8.92 g/cm 3 . The order of filling the orbitals with electrons in the Cu atom is an exception to the rule. Expected electronic configuration. 1s2 2s2 2p6 3s2 3p6 4s2 3d9. But in reality, one electron moves from ...
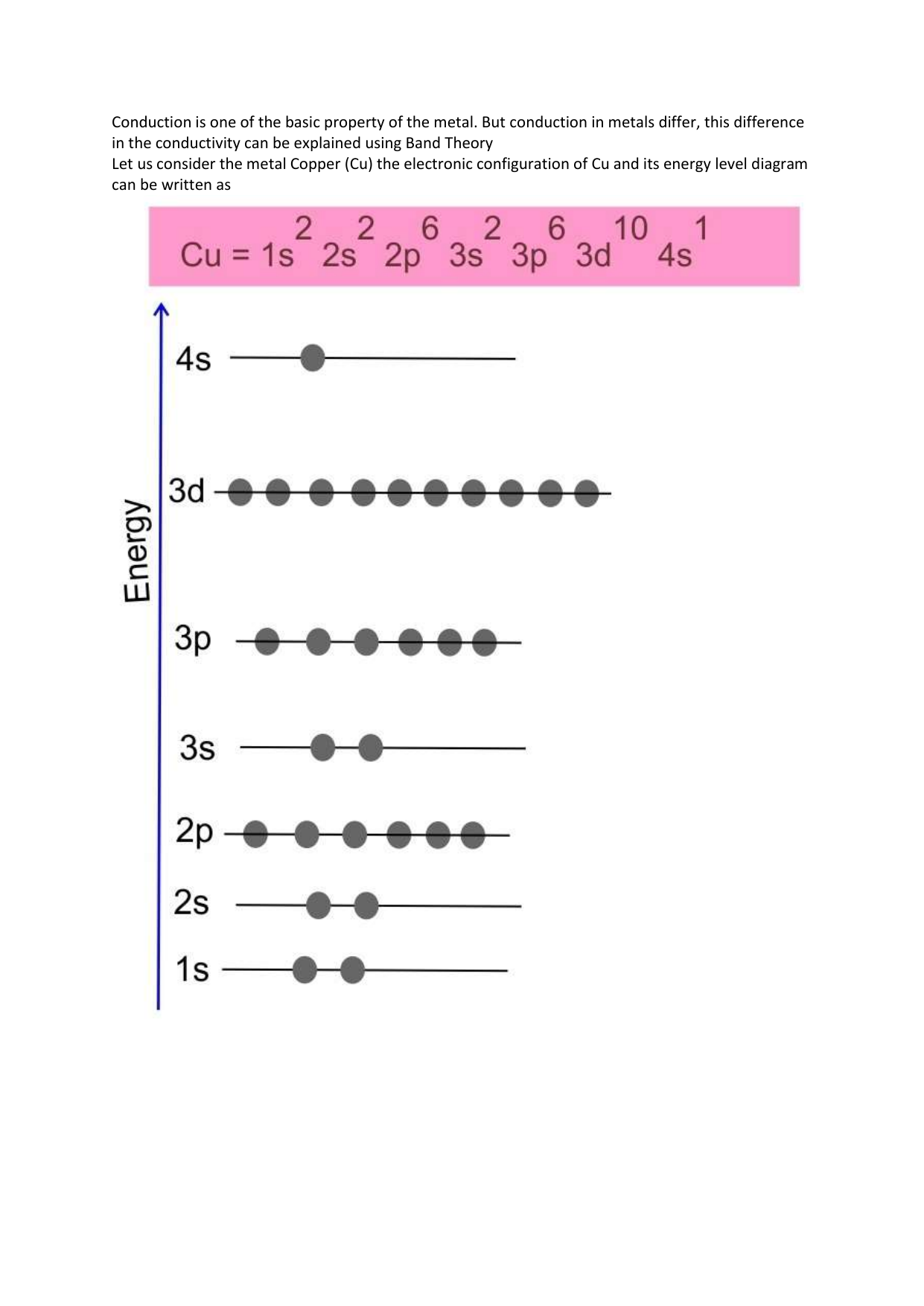
Orbital diagram for copper. the main features of molecular orbital theory for metal complexes are as follows: 1.the atomic orbital of the metal center and of surrounding ligands combine to form new orbitals, known as molecular orbitals. 2.the number of molecular orbitals formed is the same as that of the number of atomic orbitals combined. 3.the additive overlap results in … I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c... When doing the electron configurations for these elements, they are exceptions to the general rule because a completely full or half full d sub-level is more stable than a partially filled d sub-level, so an electron from the 4s orbital is excited and rises to a 3d orbital. Copper (Cu) electron configuration and orbital diagram Copper (Cu) is the 29th element in the periodic table and its symbol is 'Cu'. The electron configuration of copper and the orbital diagram is the main topic in this article….
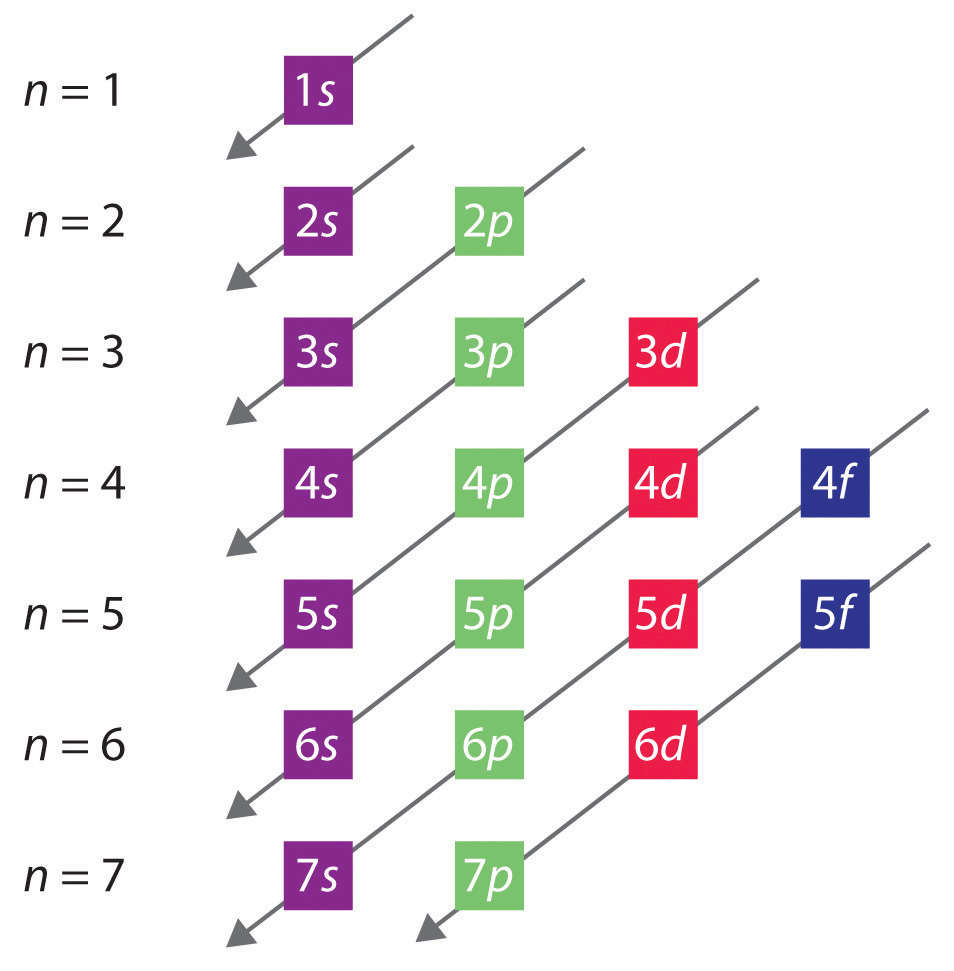
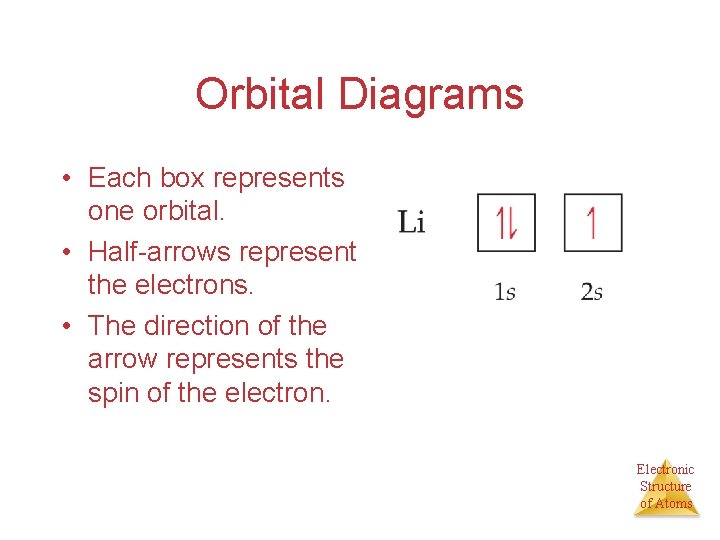
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s. So based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital. BUT what we haven't discussed is how these orbitals get filled...the order of fill. Order of Fill The ratio of the average mass per atom of an isotope to 1/12 the mass of a carbon-12 atom. Relative atomic mass is also known as atomic weight (symbol: A r ). Notes (Cu) m: Standard Atomic Weight. Cu: 63.546 (3) Isotopic Composition 63 69.15% 63 69.15% 65 30.85% 65 30.85%. Explanation: And you find that for copper, Zthe atomic number = 29. Now Z represents the CHARACTERISTIC number of protons, massive, positively-charged nuclear particles that are found in an element's nucleus. For copper Z = 29.
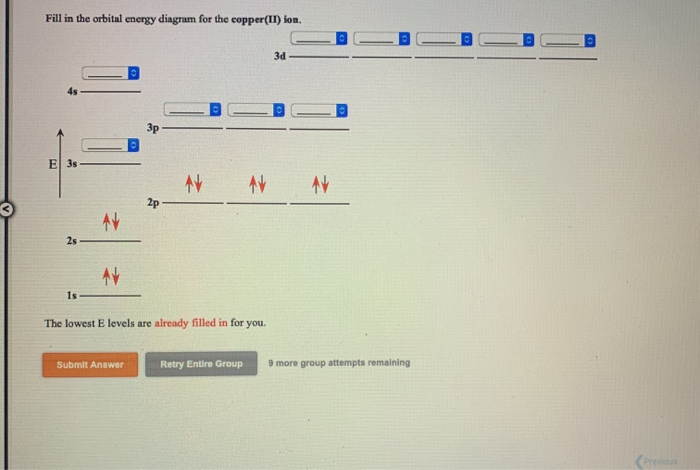
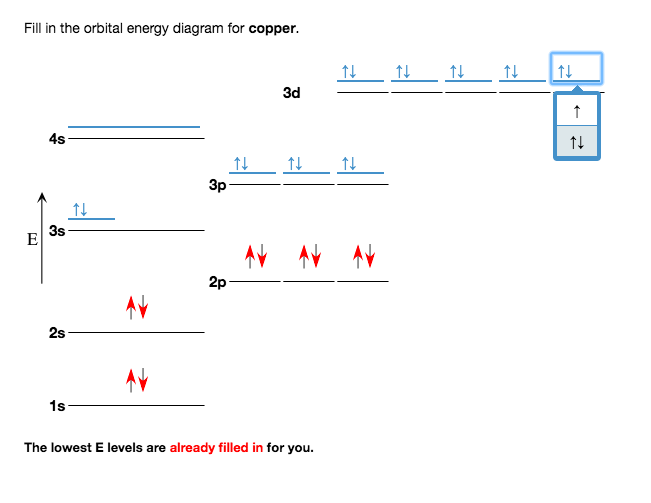
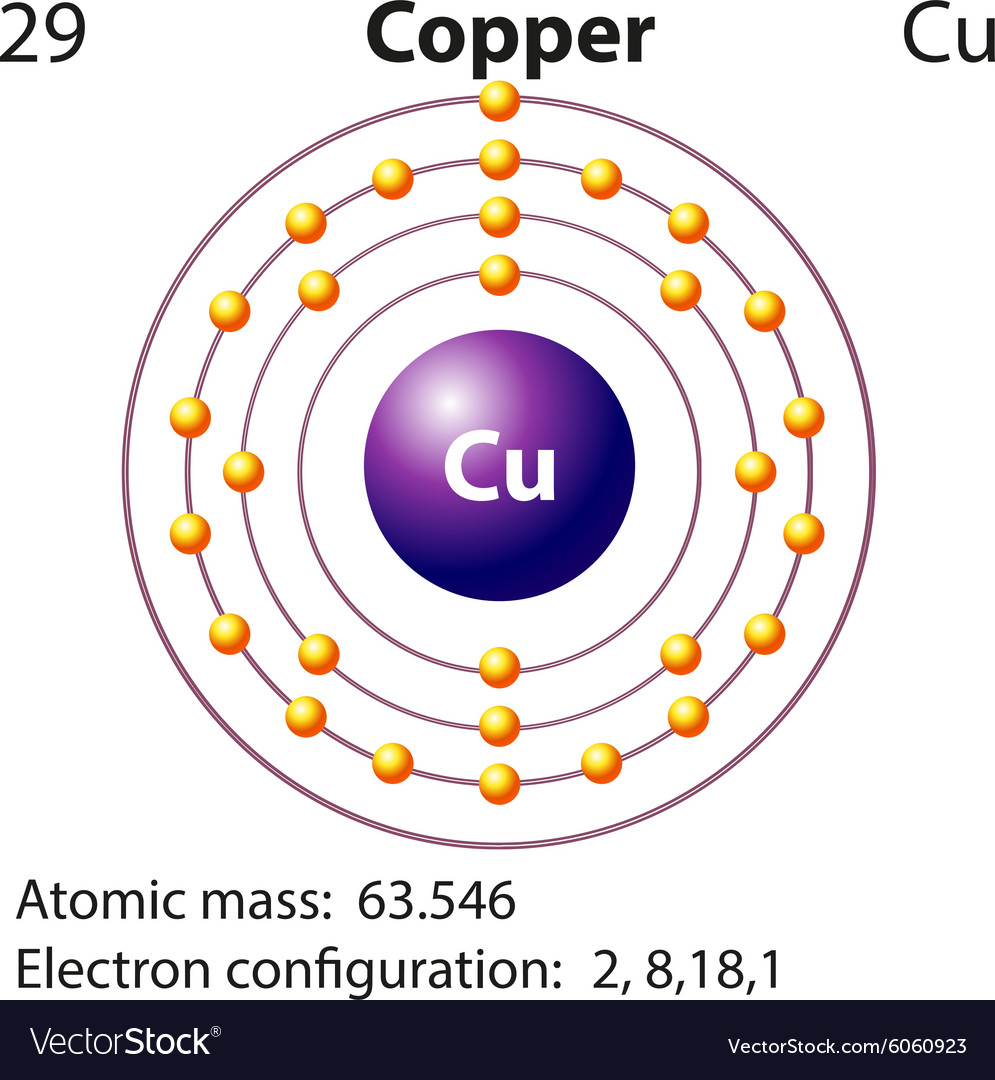
We're being asked to fill the orbital energy diagram for copper.. Before we can do that, we have to first identify the number of electrons. • In a neutral atom: Atomic number = # of protons = # of electrons Cu: atomic number = 29 → 29 protons & 29 electrons • Distribute electrons in the atomic orbitals: Start by drawing its orbital notation for the outermost, valence electrons. [Ne] ↑↓ ↑↓ ↑ ↑ 3s 3p Sulfur is a nonmetal and tends to gain electrons, creating the -2 charge. Gaining two electrons gives it an octet of 3s23p6. • Copper has two common oxidation states, +1 and +2. Atomic Orbital Diagram for Copper (Cu) Copper ion(Cu +,Cu 2+) electron configuration. Ground state electron configuration of copper(Cu) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. The electron configuration shows that the last shell of copper has an electron and the d-orbital has a total of ten electrons. In this case, the valence electrons of copper are one. There are two types of copper ions. Copper (Cu). Diagram of the nuclear composition and electron configuration of an atom of copper-63 (atomic number: 29), the most common isotope of this element. The nucleus consists of 29 protons (red) and 34 neutrons (blue). 29 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
Can u also elaborate the concept of s block p block elements
Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38:
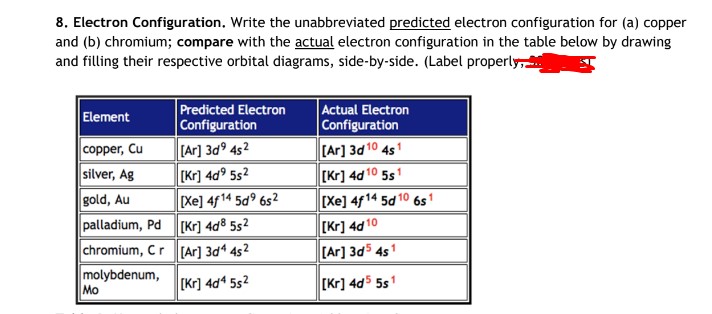
Chemistry questions and answers. a) Write the full electron configuration for Copper. b) Write the full orbital diagram for Copper. c) Write the abbreviated electron configuration for Copper. d) How many valence electrons does Copper have? Question: a) Write the full electron configuration for Copper. b) Write the full orbital diagram for Copper.
Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s orbital. The diagram shows the number of subshell by using boxes or lines for electrons use three for p orbitals five for d orbitals and 7 for f orbitals. All three ways are useful.
orbitals have one electron. Then additional electrons enter each orbital until 2 . electrons are in each orbital. Once all orbitals in a sublevel are filled (each with 2 . electrons), the next electron enters the next higher energy sublevel. The Aufbau diagram below illustrates the order of filling orbitals and sublevels.
Rhodium (Rh) electron configuration and orbital diagram. Rhodium (Rh) is the 45th element in the periodic table and its symbol is 'Rh'. The electron configuration of rhodium and the orbital diagram is the main topic in this article. Also, valency and valence electrons of rhodium, compound formation, bond formation have been discussed.
This configuration, which is at odds with the simple mnemonic, would be predicted successfully by the orbital ordering for copper given in an orbital energy diagram. Even with our best calculations, however, we can't successfully predict electron configurations for all elements using the ordering of orbital energies.
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
can u also just elaborate me the concept of s block and p block elements
Sorry if it's a dumb question, I'm having trouble understanding
Hey Guys, ​ I was wondering if there are some databases for molexular orbital diagrams of more unusual compounds like phosphaalkenes or sulfur nitrides. I wanted to include some in a presentation ​ thanks for any help!
For group, there should be a fully filled s sublevel and two electron in the outermost p sublevel. Based on how covalent bonds form with singly filled orbitals, in group14, there are only 2 singly filled orbitals respectively, how can they form the expected 4 bonds? Or does the electron from s move to p to give 4 singly filled orbitals? If that is the case, why does this happen for no reason?
Cu (Copper) is an element with position number 29 in the periodic table. Located in the IV period. Melting point: 1083.5 ℃. Density: 8.92 g/cm 3 . The order of filling the orbitals with electrons in the Cu atom is an exception to the rule. Expected electronic configuration. 1s2 2s2 2p6 3s2 3p6 4s2 3d9. But in reality, one electron moves from ...
Jun 14, 2012 · The electron configuration of copper is 1s22s22p63s23p63d104s1.
Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the next six electrons.



















/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)
0 Response to "38 orbital diagram for copper"
Post a Comment