38 lewis diagram for no2
Answer to: Draw Lewis structures for NO2+, NO2-, and NO3-. Based on the interpretation of the Lewis structures, put these in order of increasing... LEWIS DIAGRAMS The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improve-ment of Postsecondary Education (FIPSE), United States Department of Education. ... A. Free radicals such as NO and NO2. B. Some small atoms: B, Be, and Al which do not have room for a full octet in some molecules.
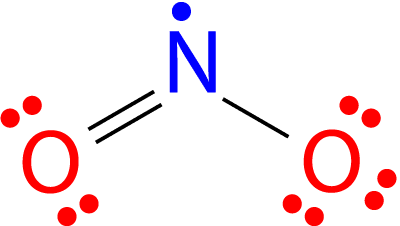
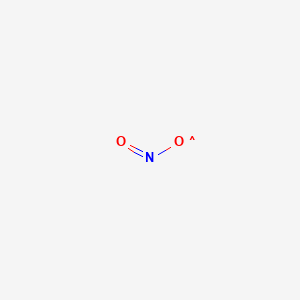
Finally, What is the Lewis structure for NO2?, Drawing the Lewis Structure for NO. This will mean that it will only have 7 valence electrons. In the Lewis structure for NO2 the Nitrogen atom is the least electronegative atom and goes at the center of the structure.For the NO2 Lewis structure, calculate the total number of valence electrons for the NO2 molecule.
Lewis diagram for no2
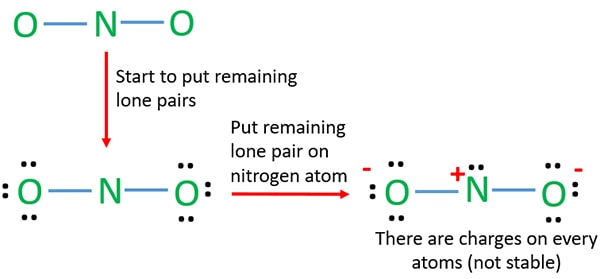
Lewis Structure of NO2. A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Let us look at the periodic table. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5. Oxygen belongs to group 16 ( or group 6) and has an atomic number of 8, therefore a valency of 6. Lewis Structure for NO 2-(Nitrite ion). Lewis structure of NO 2-ion is drawn in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge also should be considered in the drawing of NO 2-lewis structure.. Now, we are going to learn, how to draw this lewis structure. Step 1: Uselewis structure guidelines to draw the lewis structure of NO 2. Step2: Apply VSEPR notation, A X E A=Number of central atoms X=Number of surrounding atoms E= Number of lone pairs on central atom For the above molecule VSEPR notation will be AX 2 E 1. Step 3: Use VSEPR table to find the shape. AX 2 E has angular/bent shape.
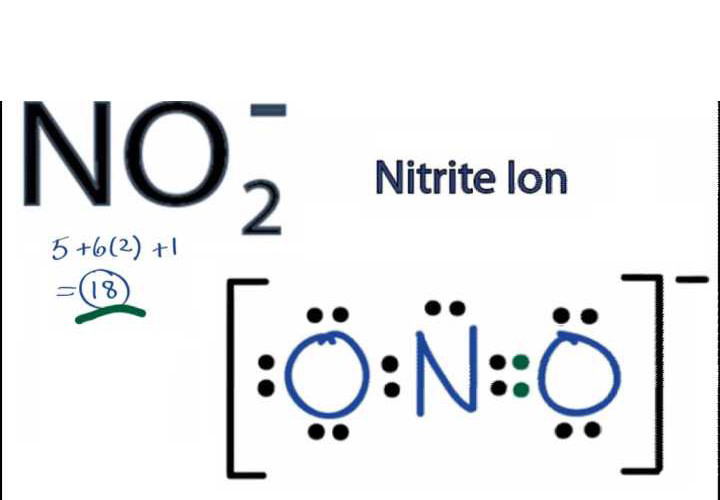
Lewis diagram for no2. A step-by-step explanation of how to draw the NO2 Lewis Structure (Nitrogen Dioxide). The NO2 Lewis structure has a total of 17 valence electrons. It's n... The nitrite ion, which is NO2(-1), has two oxygen atoms connected to a central nitrogen atom. To satisfy the octet on nitrogen, exactly ONE of the oxygens ne... This chemistry video tutorial explains how to draw the lewis structure of NO2 also known as Nitrogen Dioxide.My Website: https://www.video-tutor.netPatreon... Drawing the Lewis Structure for NO 2-(Nitrite Ion) . Viewing Notes: The Lewis structure for NO 2-(Nitrite Ion) comes up quite often in chemistry.; Be sure to put brackets, along with a negative sign, around the NO 2-Lewis structure when you are done to show that it is an ion with a negative charge.; NO 2-has a total of 18 valence electrons.
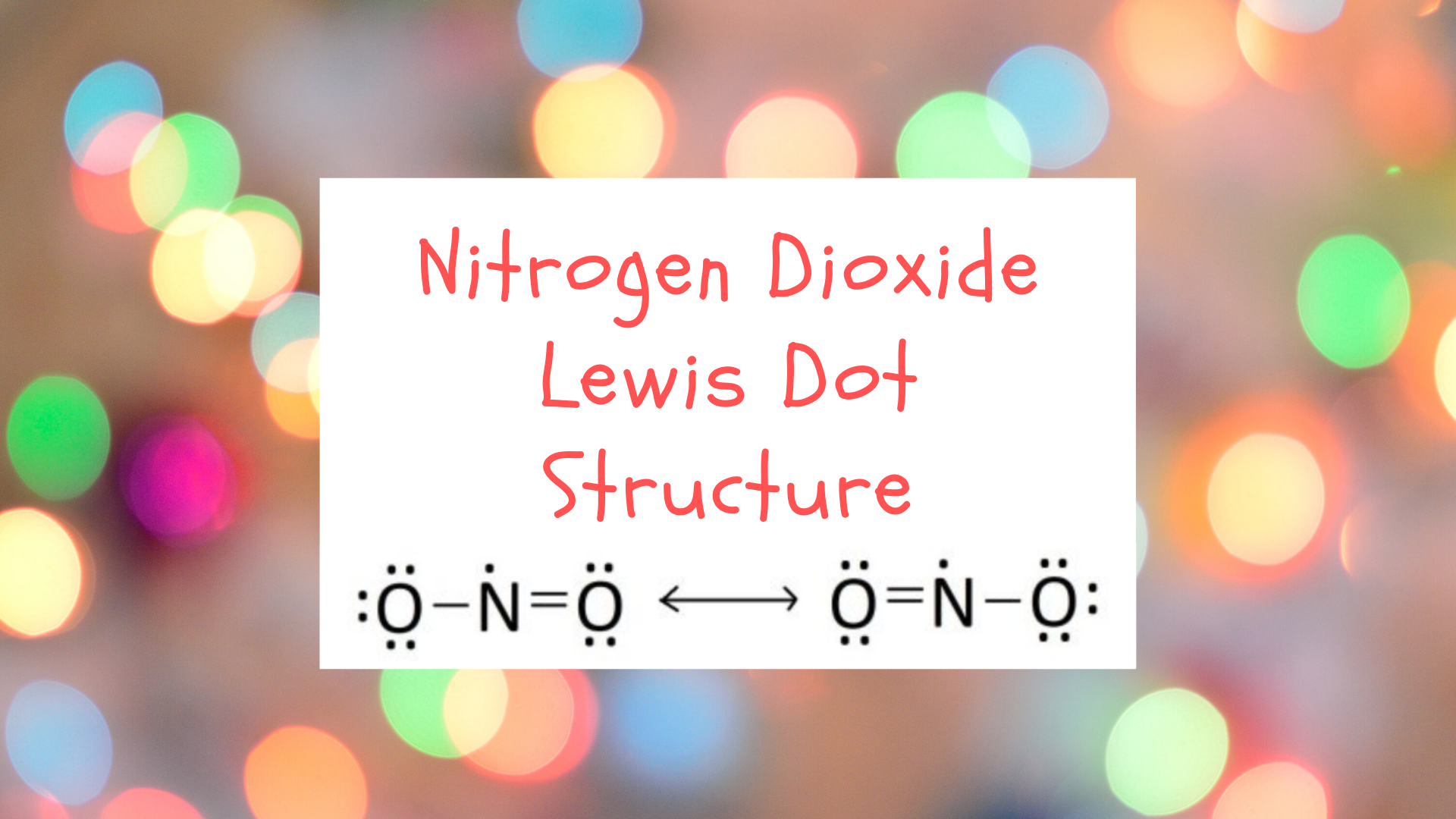
NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3. Part B : Draw the Lewis structure of NO2−. Part C: Assign formal charges to each atom in the O3 molecule Part D:Based on formal charges, draw the most preferred Lewis structure for the chlorate ion, ClO3−? Best Answer. This is the best answer based on feedback and ratings. 95% (19 ratings) NO2F Lewis Structure, Molecular Geometry, Hybridization, and Polarity. NO2F, an inorganic compound, is named Nitryl Fluoride. It is a colorless gas and acts as a strong oxidizing agent. So, it can be used in the replacement of liquid oxygen, an oxidant in propellants of the rocket. NO2F can easily release its fluoride ion to other species in ... For the detailed electronic structure of NO2, you must read the article on NO2 Lewis Structure, Geometry, Hybridization. Factors affecting polarity of a molecule. If you want to check the polarity of a molecule, you just need to know the below three points about that molecule. Let us below check them what they mean actually.
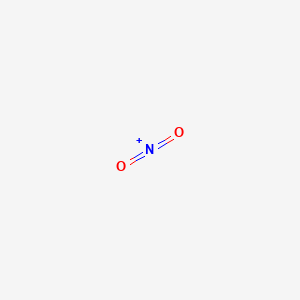
For the NO2- Lewis structure, calculate the total number of valence electrons for the NO2- molecule. After determining how many valence electrons there are in NO2-, place them around the central atom to complete the octets. There are a total of 18 valence electrons for the Lewis structure for NO2-. Nitrogen is the least electronegative atom in ... NO2+ lewis structure "NO2+ lewis structure" explain with this techniques, First of all, you find the number of balance electron of (NO2+) ion. You know that, Nitrogen (N) has 5 balance electron & Oxygen (O) has 6 balance electron. Now, the overall charge of NO2+ ion has +charge (NO2+). so, it reduce number of electron by (-1). Its bond order is 2. If you mean NO2+, the MO diagram of NO is: Thus, NO2+ loses the 2b1 antibonding electron and the 3a1 bonding electron, and its bond order is around 2.5. This does not match the given answers, but you had left that possibility open. If you mean NO+ 2, it is isoelectronic with CO2, whose bond order is 2. In lewis structure NO 2-ion, there are three lone pairs (in the last shell) in one oxygen atom and that oxygen atom is joint with nitrogen atom by a single bond. Also, that oxygen atom has a -1 charge. What is the Lewis structure of ClO2? Drawing the Lewis Structure for ClO.
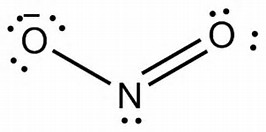
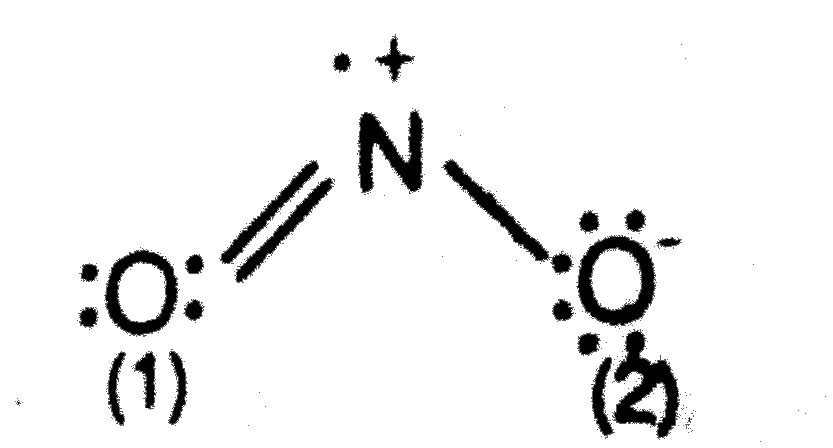
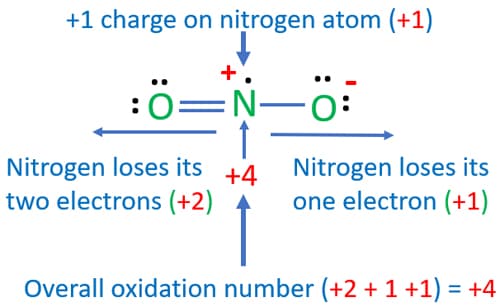
Lewis Structure for NO 2 | Nitrogen dioxide Oxidation number. Lewis structure of NO 2 (Nitrogen dioxide) is drawn in this tutorial step by step. You can learn basics of how to draw a lewis structure properly from this example. This is a special case of lewis structure drawing because, there is a unpaired electron on nitrogen atom.
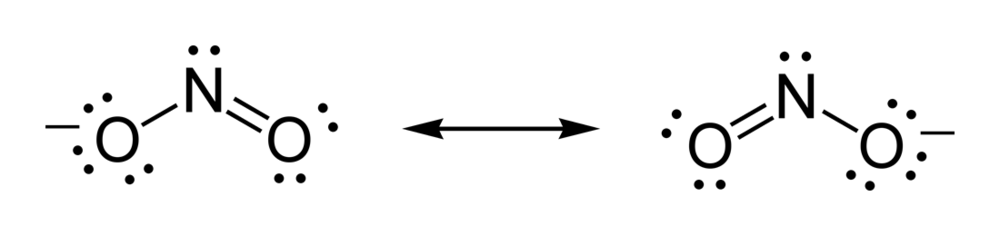
NO 2-Resonance Structures (Nitrite ion). When we drew lewis structure of NO 2-ion, we can draw resonance structures of NO 2-ion. In this tutorial, you can see how many resonance structures can be drawn for nitrite ion (NO 2-) and what are the steps you need to know.Also, we will discuss which resonance structures are more stable an which are less stable.
In the Lewis structure for NO2 the Nitrogen atom is the least electronegative atom and goes at the center of the structure. For the NO2 Lewis structure, calculate the total number of valence electrons for the NO2 molecule. After determining how many valence electrons there are in NO2, place them around the central atom to complete the octets.
What is the Lewis structure of NO2 - Wolfram|Alpha. Volume of a cylinder? Piece of cake. Unlock Step-by-Step. Natural Language. Math Input.
The NO2 Lewis structure has a total of 17 valence electrons. It's not common to have an odd number of valence electrons in a Lewis structure. Because of this we'll try to get as close to an octet as we can on the central Nitrogen (N) atom. This will mean that it will only have 7 valence electrons.
A step-by-step explanation of how to draw the NO+ Lewis Dot Structure (Nitronium ion).For the NO+ structure use the periodic table to find the total number o...
Incomplete Lewis structures for the nitrous acid molecule,HNO2, and the nitrite ion, NO2^-, are shown below. a) complete each Lewis structure by adding electron pairs as needed. b) s the formal charge on N the same or different in these two species? c) would either HNO2 or NO2^- be expected to...
View this answer. The following steps lead to the Lewis structure for NO 2 . 1. Total number of valence electrons: The number of valence electrons in nitrogen is five... See full answer below.
Please find the Lewis Dot Structure for NO2+ (also known as nitronium ion) shown above. As with all other Lewis Dot Structures the bonds within the structure can be replaced by two dots. Both of the oxygen's on the ends of NO2+ contain two lone pairs of electrons and are chemically neutral.
A step-by-step explanation of how to draw the NO2 - Lewis Dot Structure (Nitrite ion).For the NO2 - structure use the periodic table to find the total number...
Step 1: Uselewis structure guidelines to draw the lewis structure of NO 2. Step2: Apply VSEPR notation, A X E A=Number of central atoms X=Number of surrounding atoms E= Number of lone pairs on central atom For the above molecule VSEPR notation will be AX 2 E 1. Step 3: Use VSEPR table to find the shape. AX 2 E has angular/bent shape.
Lewis Structure for NO 2-(Nitrite ion). Lewis structure of NO 2-ion is drawn in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge also should be considered in the drawing of NO 2-lewis structure.. Now, we are going to learn, how to draw this lewis structure.
Lewis Structure of NO2. A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Let us look at the periodic table. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5. Oxygen belongs to group 16 ( or group 6) and has an atomic number of 8, therefore a valency of 6.




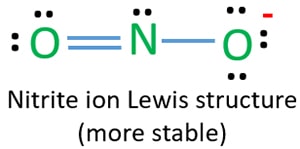






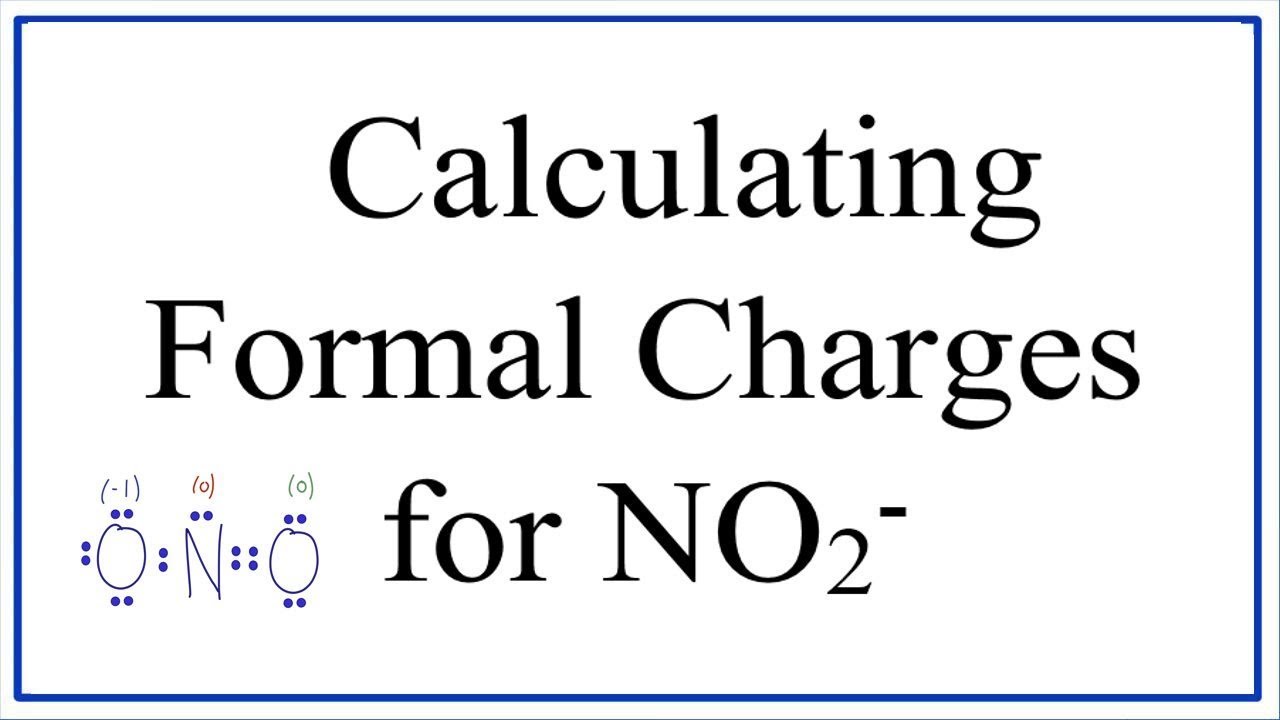

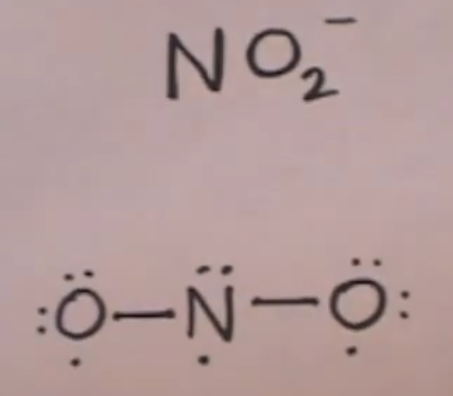











0 Response to "38 lewis diagram for no2"
Post a Comment