36 maximum solubility phase diagram
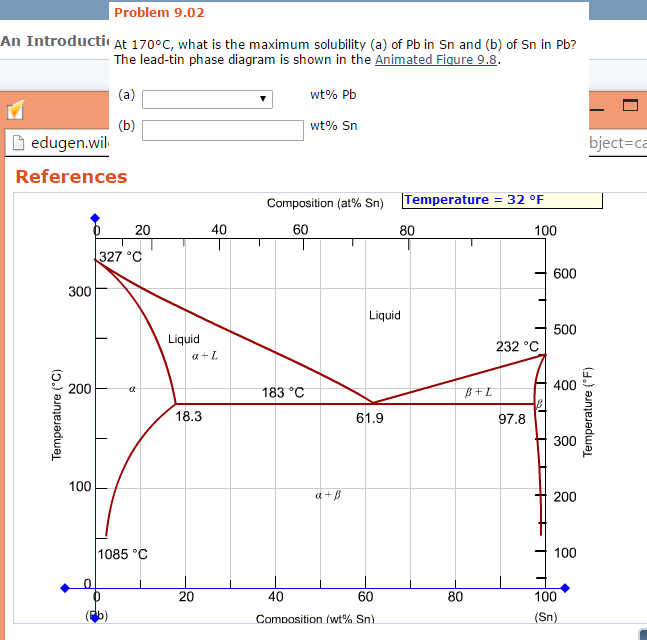
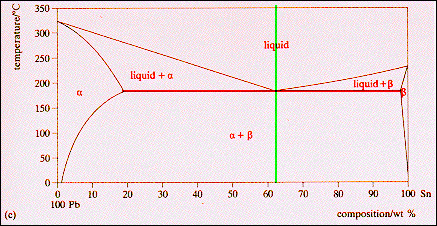
The maximum solid solubility of tin in lead occurs at the eutectic temperature (183 C) and the ... -phase at this temperature has the composition Pb-19.2 wt % Sn. Similarly, at the tin rich end of the composition range, lead has its maximum solid solubility in tin at the eutectic temperature and the At 70°C, what is the maximum solubility (a) of Pb in Sn and (b) of Sn in Pb? The lead-tin phase diagram is shown in the Animated Figure 9.8 (a) wt% Pb (b) [The tolerance is +/- 20%. wt% Sn Composition = Composition = Temperature Temperature Composition (at% Sn) 40 60 100 327 °C 600 300 Liquid 500 Liquid 232 °C a L 400 200 183 °C B+L 18.3 61 ...
Thinking of binary phase diagrams in terms of solubility limits.
Maximum solubility phase diagram
Hardness of pure material is very poor and maximum strength will be at the point of maximum solid solubility. Influence of Alloying Elements on Phase Diagram: Alloys elements addition always reduces eutectoid composition i.e.% of C while they may increase or decrease the eutectoid temperature. This page contains materials for the solid solutions class session introducing binary phase diagrams with a focus on the complete solubility case. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with ... Fig 1 Iron carbon phase diagram. C is an interstitial impurity in Fe. It forms a solid solution with alpha, gamma and delta phases of iron. Maximum solubility of C in alpha iron is 0.025 % at 727 deg C. Body centred cubic (BCC) iron has relatively small interstitial positions. Maximum solubility of C in the face centred cubic (FCC) gamma iron ...
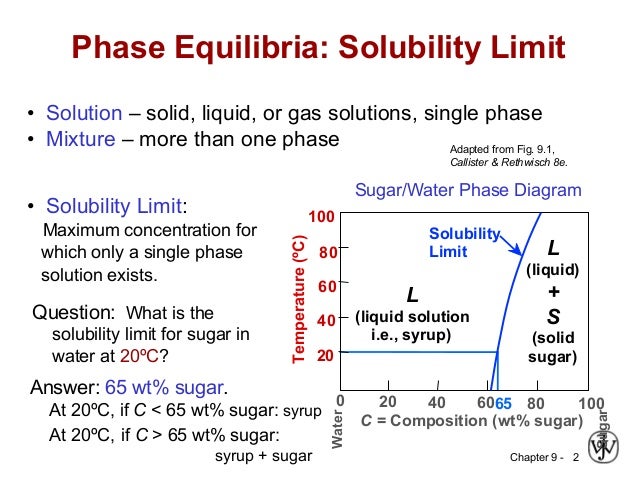
Maximum solubility phase diagram. Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on YouTube. solidification are like in a single component diagram; The melting temperature is the lowest for the Eutectic composition. •Max Solubility Limit-is the max amount of component b in α/ max amount of component a in b. The max solubility limit is always at the eutectic temperature. The extent of solid solubility of phases, the stability of phases, the temperature dependence of stability, and the choice of structures that are actually observed in phase diagrams are the result of competition among numerous possible structures that could be stable in a given system. Phase equilibria: solubility limit Introduction –Solutions – solid solutions, single phase –Mixtures – more than one phase • Solubility Limit: Max concentration for which only a single phase solution occurs. Sucrose/Water Phase Diagram Sugar) 0 20 40 60 80 100 C o=Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility ...
Cellulose solubility phase diagram (shaded areas) in EmimAc-DMSO (line corresponds to 2.5 EmimAc molecules bound to 1 AGU) and in EmimAc-water (line corresponds to experimental results) with dashed line being a possible continuation of C cell max in EmimAc-water. Concentrations are in wt%. Welcome to Materials Science · This course provides experiences in exploring materials through self-directed, hands-on projects. I hope the course environment works well for you, and I hope that you find your time in Materials Science and Solid State Chemistry interesting and enjoyable. Lecture Notes for MSE 2090-1 · University of Virginia, Department of Materials Science and Engineering MSE 2090: Introduction to the Science and Engineering of Materials Fall 2010 MSE 2090 - Section 1, Monday and Wednesday, 08:30 - 9:45 am, Olsson Hall 009 · Notes in pdf format Notes in pdf ... The solubility limit of Ag in (Cu) is 7.9 wt. % Ag. The solubility limit of Cu in (Ag) is 8.8 wt.% Cu. Note that these. Maximum solubility phase diagram. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagram s 3 Solvent - host or major component in solution,solute- minor component. Solubility Limitof a component in a phase is the ...
MSE 170 Fundamentals of Materials Science · Instructor: Prof. Brian Flinn, Prof Y. K. Rao November 3, 2009 - Our mission is to lead materials information technology—to advance materials engineering and education, and to enable better, greener, safer products. June 28, 2018 - Ceramics were the first artificial material utilized by mankind. Since the dawn of civilization, polycrystalline non-metallic materials, that now we called ceramics, occupy very important but ever changing place in our life. Ceramic thin films used a very large range of every day-use devices ... UC's College of Engineering and Applied Science is the birthplace of cooperative education (co-op). From cyber operations to construction management, CEAS has a major for you. Learn more!
Answer (1 of 4): Before it's answer you must know about that carbon present in Fe combine with it to form Fe3C. Means we can add carbon as much as possible until all Fe is converted into Fe3C. Thus weight %of C in Fe when all Fe converted into Fe3C will be max wt% of C in Fe. For this Let us as...
NPTEL provides E-learning through online Web and Video courses various streams.
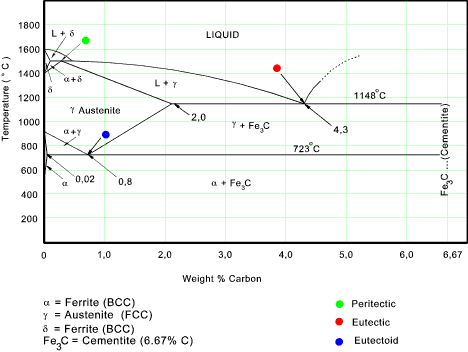
Definition of structures: Austenite is an interstitial solid solution of Carbon dissolved in (F.C.C.) iron. Maximum solubility is 2.0 % C at 1130°C. High formability, most of heat treatments begin with this single phase. It is normally not stable at room temperature. But, under certain conditions it is possible to obtain austenite at room temperature.
Phases in Fe-Fe3C Phase Diagram ¾α-ferrite - solid solution of C in BCC Fe • Stable form of iron at room temperature. • The maximum solubility of C is 0.022 wt% • Transforms to FCC γ-austenite at 912 °C ¾γ-austenite - solid solution of C in FCC Fe • The maximum solubility of C is 2.14 wt %. • Transforms to BCC δ-ferrite at ...
alpha starts to precipitate out · beta starts to precipitate out
13.2 Phase Diagrams: Binary Systems. As explained in Sec. 8.2, a phase diagram is a kind of two-dimensional map that shows which phase or phases are stable under a given set of conditions. This section discusses some common kinds of binary systems, and Sec. 13.3 will describe some interesting ternary systems.
How to read the phase diagram. ... The solvus line shows that the host substance A has its maximum solubility at a temperature of 700 °C and can store 20 % B atoms. At this temperature, the entire B atoms of the alloying element remain dissolved in the lattice structure of the host material A.
The maximum solubility of Y in α-Mg and composition ranges of Mg24Y5 − x and Mg2Y1 − x intermetallic phases in Mg–Y binary system - ScienceDirect
Phase Diagrams This common tangent construction can be extended to quite complicated situations, with several minima, which then give rise to quite complicated free energy curves and hence phase diagrams. For plotting a phase diagram we need to know how solubility limits (as determined by the common tangent construction) vary with temperature.
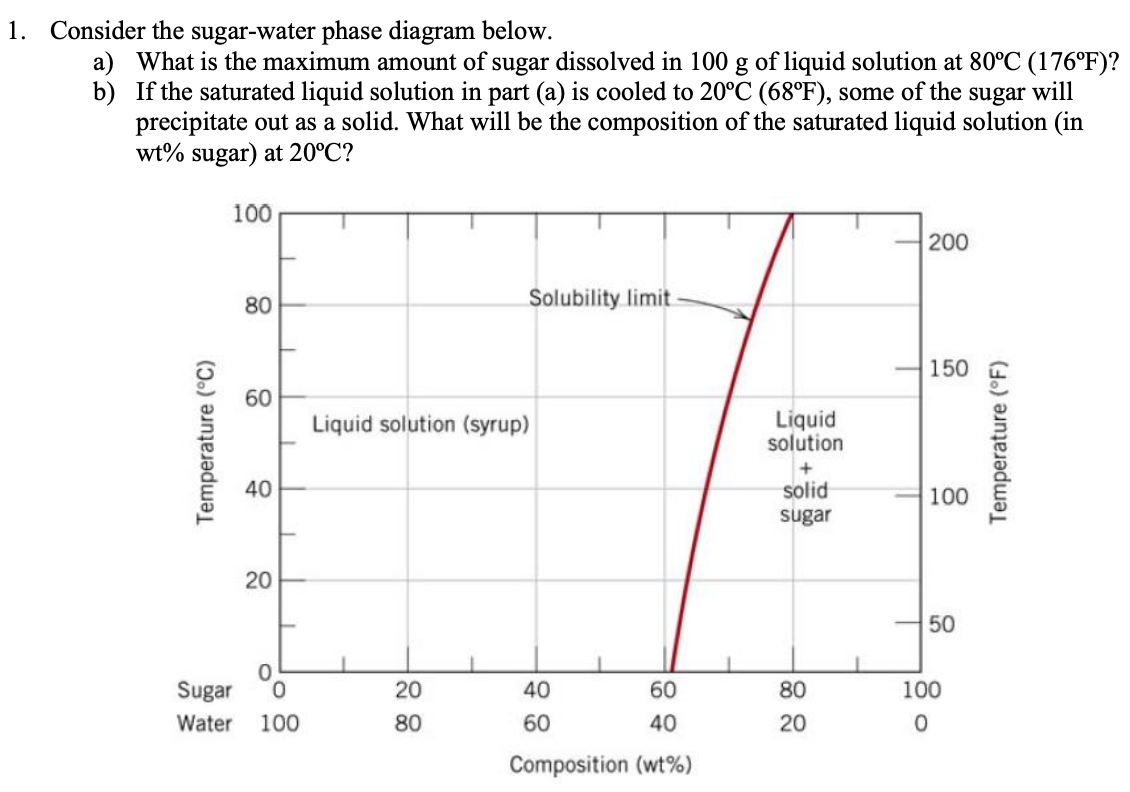
2 • Solubility Limit: Max concentration for. which only a solution. occurs. • Ex: Phase Diagram: Water-Sugar System. Question: What is the. solubility limit at 20C?
Chapter 9: Phase Diagrams Phase A Phase B Nickel atom Copper atom. Chapter 9 - 2 Phase Equilibria: Solubility Limit Introduction – Solutions – solid solutions, single phase – Mixtures – more than one phase • Solubility Limit : Max concentration for which only a single phase solution occurs. Question: What is the solubility limit at 20°C?
Compositions run from 100% Element A on the left of the diagram, through all possible mixtures, to 100% Element B on the right. The composition of an alloy is given in the form A - x%B. For example, Cu - 20%Al is 80% copper and 20% aluminium · Weight percentages are often used to specify the ...
MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 3 Solvent - host or major component in solution,solute- minor component. Solubility Limitof a component in a phase is the maximum amount of the component that can be dissolved
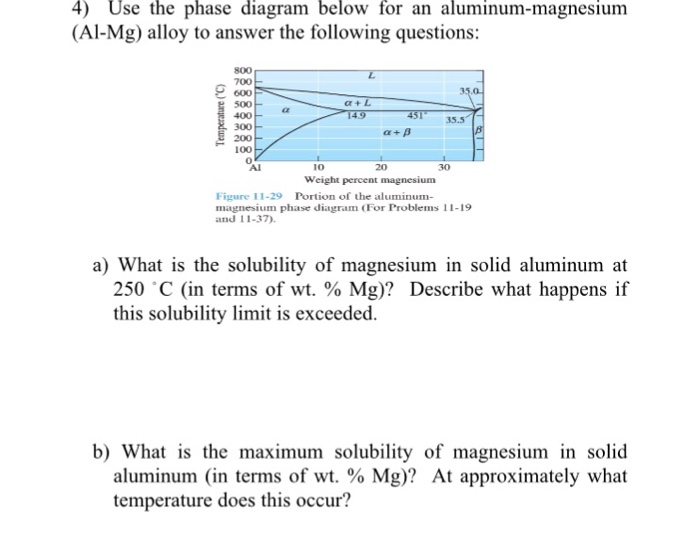
+ β) phase boundary at this temperature, or to about 2 wt% Cu. (b) From this same figure, the maximum solubility of Ag in Cu corresponds to the position of the α–(α + β) phase boundary at this temperature, or about 1.5 wt% Ag. 9.8 Cite the phases that are present and the phase compositions for the following alloys:
Chapter 9. Phase Diagrams. [ Up ] [ Chapter 6. Mechanical Properties of Metals ] [ Chapter 7. Dislocations and Strengthening Mechanisms ] [ Chapter 8. Failure ] [ Chapter 9. Phase Diagrams ] [ Chapter 10: Phase Transformations in Metals ] [ Chapter 11. Thermal Processing of Metal Alloys ] [ ...
The Al-Si Phase Diagram The binary Al-Si phase diagram was initially studied by Fraenkel of Germany in 1908. It is a relatively simple binary diagram where there is very little solubility at room temperature for Si in Al and for Al in Si. Thus, the terminal solid solutions are nearly pure Al and Si under equilibrium conditions.

(Fang Ruida) Davis. K Coronavirus pneumonia biomarkers and treatment(方瑞达) Davis。K 冠状型病毒性肺炎生物标志物及治疗防治
The maximum solid solubility on a phase diagram is controlled by factors described by _____ Hume-Rothery's rules. The proeutectoid solid phase for a hypoeutectoid alloy is _____. (Look at the FeC diagram). ferrite. The melting reaction that occurs in an alloy system in which there is no change in ...
According to Murray the assessed Mg-Al phase diagram consists of liquid, -solid solution with hexagonal crystal structure, -solid solution with the αMn structure type, R phase with rhombohedral structure at 42 at.% Mg, Al solid solution with a maximum solubility of 18.9 at.%
This phase is a solid solution of carbon in FCC Fe with a maximum solubility of 2.14% C. On further heating, it converts into BCC δ-ferrite at 1395°C. γ-austenite is unstable at temperatures below eutectic temperature (727°C) unless cooled rapidly.
https://goo.gl/nMe9J1 For 60+ videos on Engineering Materials
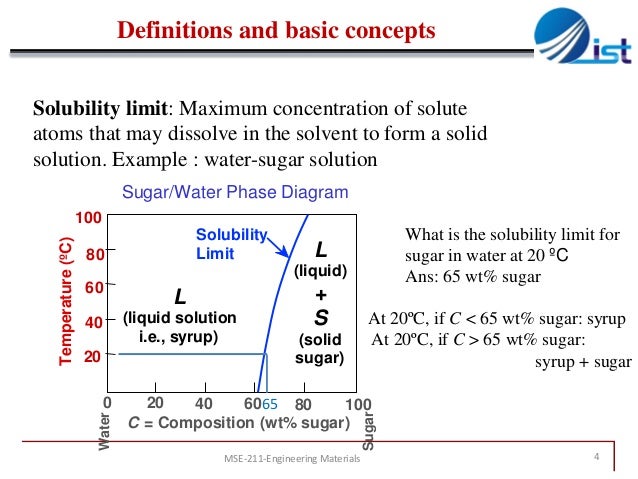
The Solubility Limit • Max. concentration for which only a solution occurs. -if Co < 65wt% sugar: syrup-if Co > 65wt% sugar: syrup + sugar • Solubility limit increases with T -e.g ... Phase Diagrams • Information about phases as function of T, Co, P • For this course:
At 100 C, what is the maximum solubility (a) of Pb in Sn? (b) of Sn in Pb? The lead-tin phase diagram is shown below. Round to the nearest whole number. Question: At 100 C, what is the maximum solubility (a) of Pb in Sn? (b) of Sn in Pb? The lead-tin phase diagram is shown below. Round to the nearest whole number.
The maximum temperature at which the two phase region exists is called the critical solution temperature. The critical solution temperature in this experiment is 70˚C. During this experiment, the temperature of the phenol-water system at miscible and temperature at which two phases separated is measured.
Solubility Limit of a component in a phase is the maximum amount of the component that can be dissolved in it (e.g. alcohol has unlimited solubility in water, sugar has a limited solubility, oil is insoluble). The same concepts apply to solid phases: Cu and Ni are mutually soluble in any amount ...

Monochrome, Unesco World Heritage Site, National Trust, Water Landscape, Giant's Causeway, County Antrim, Northern Ireland.
The binary Pb-Sn phase diagram has been studied for over 100 years and is a classic eutectic. Lead (Pb) has an atomic number of 82 and a high density. Its crystal structure is face-centered cubic (fcc). At 50 C, 2% Sn is soluble in Pb and at the eutectic temperature, 183 C, the maximum solubility of Sn in -Pb is 19%.
Fig 1 Iron carbon phase diagram. C is an interstitial impurity in Fe. It forms a solid solution with alpha, gamma and delta phases of iron. Maximum solubility of C in alpha iron is 0.025 % at 727 deg C. Body centred cubic (BCC) iron has relatively small interstitial positions. Maximum solubility of C in the face centred cubic (FCC) gamma iron ...
This page contains materials for the solid solutions class session introducing binary phase diagrams with a focus on the complete solubility case. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with ...
Hardness of pure material is very poor and maximum strength will be at the point of maximum solid solubility. Influence of Alloying Elements on Phase Diagram: Alloys elements addition always reduces eutectoid composition i.e.% of C while they may increase or decrease the eutectoid temperature.








0 Response to "36 maximum solubility phase diagram"
Post a Comment