36 electron configuration and orbital diagram worksheet
Electron Orbitals: Electron Configuration Orbital Diagram Worksheet Answers (The electron configuration orbital diagram worksheet answers can be found at the bottom of the lesson.) The 2, 8, 8, 18 rule is a very simplistic view of electron configuration and doesn't give the full picture when it comes to electron configuration. Electron Configuration and Orbital Diagram Worksheets - On these two practice worksheets, students write full electron configurations and noble gas configurations, complete orbital diagrams, and mark valence electrons. Worksheet #1 (2 pages) - Includes notes and a walkthrough example on solving el
Advanced Placement Chemistry - Electron Configuration Worksheet. Draw the orbital diagram for the following elements: Oxygen (O) Titanium (Ti) Silicon (Si) Copper (Cu) For each of the following elements, identify if the electron configuration is correct or incorrect. If it is incorrect, give the fix to the configuration. Carbon (C) = 1s22s22p2
Electron configuration and orbital diagram worksheet
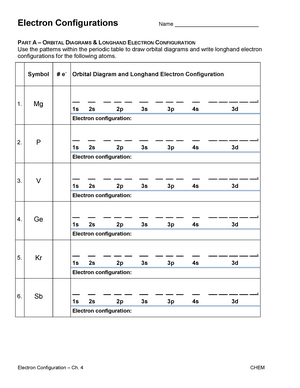
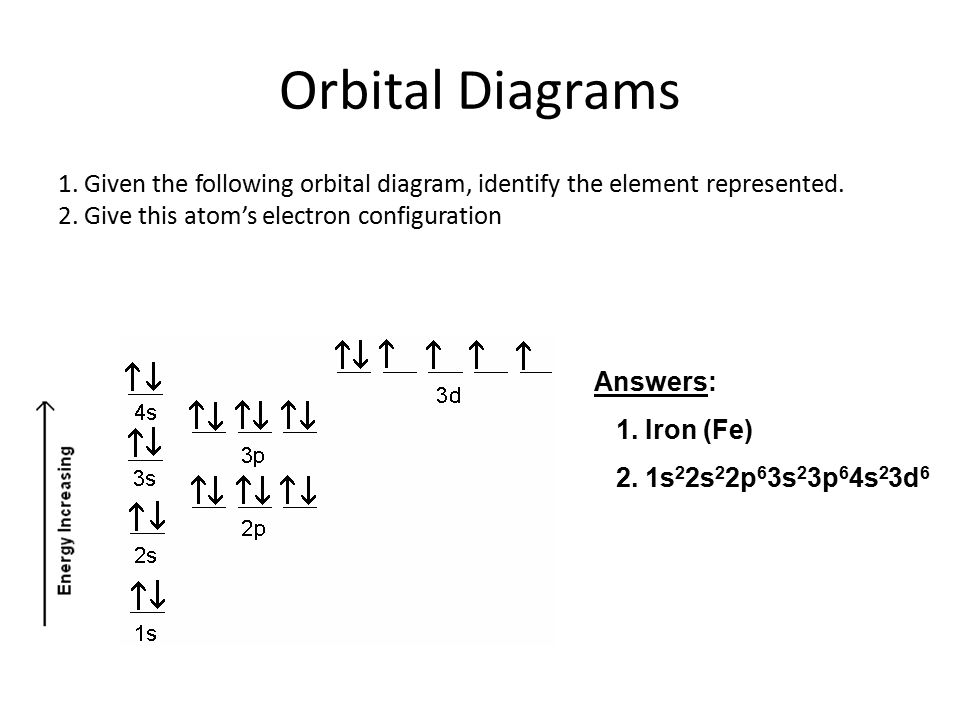
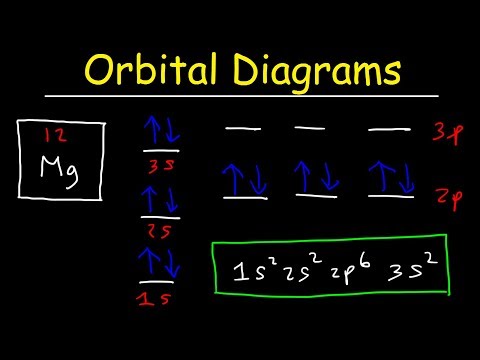
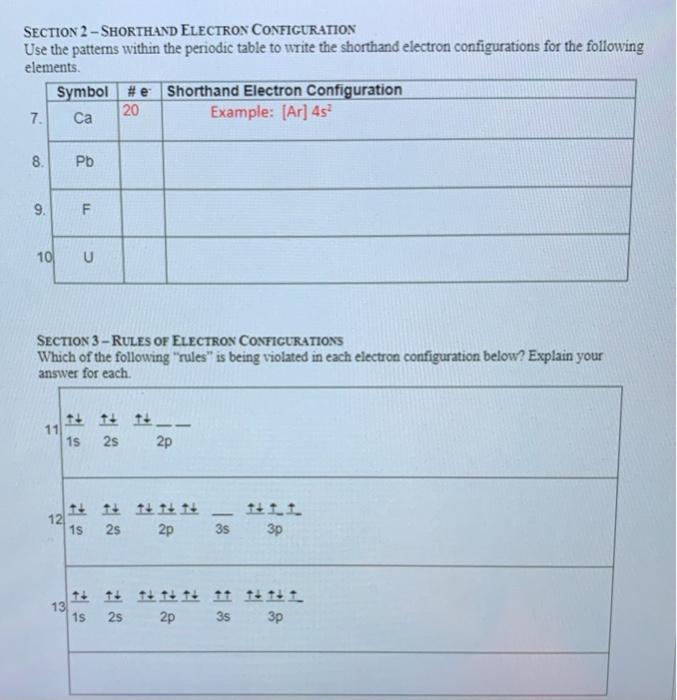
Symbol # e- Orbital Diagram and Longhand Electron Configuration 1. Mg 1s 22s2 2p6 3s 2. P 1s2 22s2 32p6 3s 3p 3. V 1s2 6 2s2 2p 3s2 3p6 4s23d3 4. Ge 1s 22s 2p63s 3p64s23d104p2 PART B – SHORTHAND ELECTRON CONFIGURATION Use the patterns within the periodic table to write the shorthand electron configurations for the following elements. Symbol # e- Shorthand Electron Configuration 5. Ca [Ar] 4s2 6. Pb [Xe]6s24f145d106p2 7. F [He]2s22p5 8. Pd [Kr]5s24d8 10. Which element has the following orbital diagram? 11. Using arrows, show how the following orbitals will fill with electrons. Electron Configuration 1s 2s 2p 3s 3p 4s 3d Mg 1s22s22p63s2 Cl 1s22s22p63s23p5 Si 1s22s22p63s23p2 Ti 1s22s22p63s23p64s23d2 12. Write the complete electron configuration for each atom on the blank line. View Electron Configurations and Orbital Diagrams Worksheet_Smart.pdf from CHM 102 at Northern Virginia Community College. Kavya Smart Calcium Ca Nickel Ni Carbon C Xenon Xe Protactinium Pa Sulfur
Electron configuration and orbital diagram worksheet. Chemistry. Chemistry questions and answers. Electron Configurations Worksheet Write the complete ground state electron configurations and orbital notations for the following: #of e Element (atom) e configuration Orbital Notations/ diagrams 1) 3 lithium 15^2 2541 [1L] [1] 2) 8 oxygen 18^2 25^2 2P44 [1L] [1L] [11] [1] [1] [11] [11] [14] [14] [14 ... Electron Configurations Worksheet Write the complete ground state electron configurations and orbital notations for the following: # of e - Element (atom) e - configuration Orbital Notations/ diagrams Electron Configuration – Ch. 4 CHEM Electron Configurations Name _____ Date _____ Per _____ PART A – ORBITAL NOTATION Use the patterns within the periodic table to write the orbital notation for the following atoms. Symbol # e-Orbital Notation 1. Mg Electron Configurations, Orbital Notation and Quantum Numbers 318 Laying the Foundation in Chemistry 5 • Transition metals generally have an oxidation state of +2 since they lose the s2 that was filled just before the d-sublevel began filling.
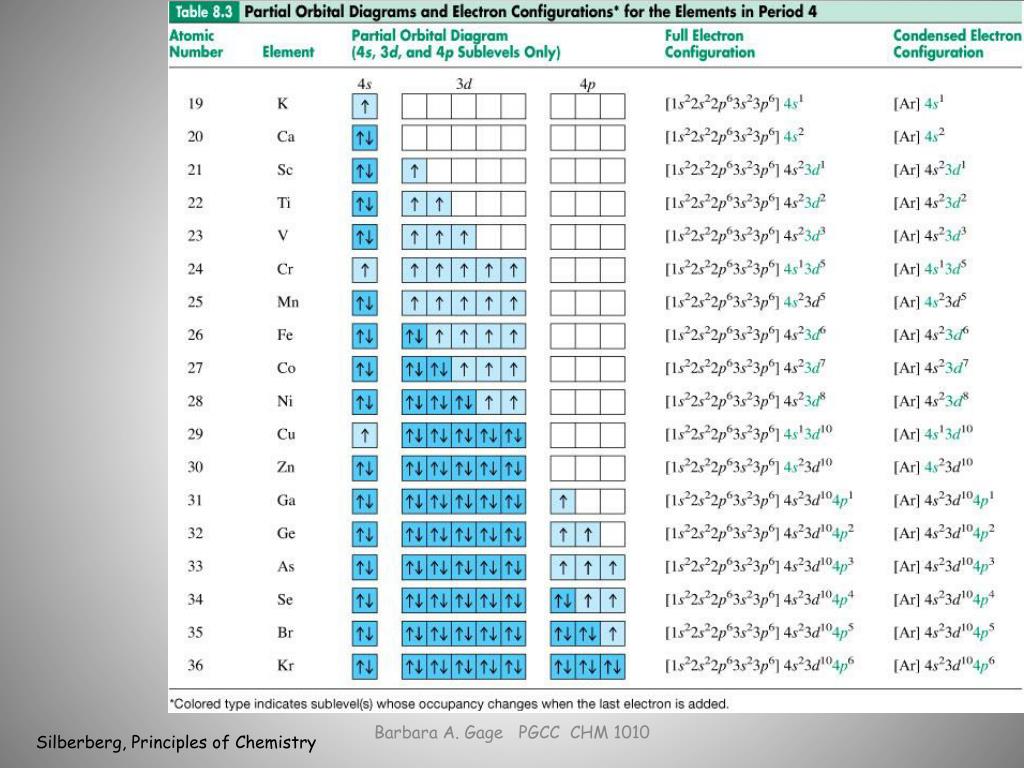
Reminder of electron configuration rules: Aufbau Principle: Electrons occupy lowest energy levels first. Pauli Exculsion principle: Each orbital contains up to 2 electrons. If an orbital contains 2 electrons, they must have opposite “spin.” Hund’s rule: One electron must occupy each orbital in a sublevel before a second electron occupies an orbital. Part B – Rules of Electron Configurations The orbital diagram in Model 3 is higher in energ than the ground state because ther is an electron in the 3 orbital that should be in a 2P orbital. The electron would need to have higher potential ennu to be in the 3s orbital. Read This! An state electron configuration is any electron configuration for an atom that contains the Electron Arrangements Name There are three ways to indicate the arrangement of electrons around an atom: 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. HW #6 - Electron Configuration and Orbital Diagrams Directions: Please fill in the electron configuration for the following elements and then draw the orbital diagram configuration in the appropriate boxes. F Electron Configuration Mg Electron Configuration Orbital Diagram Configuration Orbital Diagram Configuration B Electron Configuration He
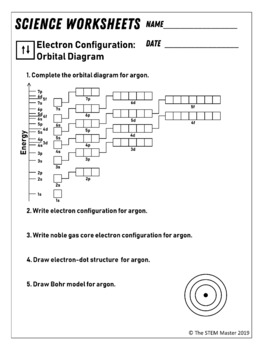
Electron configuration and orbital diagram worksheet. Electron configurations worksheet write the complete ground state electron configurations and orbital notations for the following. Phosphorus 1s 2s 2p 3s 3p 4s 3d 4p. Write orbital filling diagrams electron configurations and electron dot diagrams. O2 1s2 2s2 2p4. Chemistry Worksheet - Review for Electronic Structure of Atoms Page 3. Write the electron configuration (ie: 1s2, 2s1), orbital diagram, noble gas notation, and Lewis dot notation for: H, Li, Na, and K atoms. 2. Be, Mg and Ca atoms. 3. C, Si and Ge atoms. 4. F, Cl and Br atoms. 5. Fe, Ni and Zn atoms. 6. S -2 and K1+ ions. 7. Mg2+ and F1- ions You may be offline or with limited connectivity. ... Download An electron configuration is a method of indicating the arrangement of electrons about a nucleus. A typical electron configuration consists of numbers, letters, and superscripts with the following format: 1. A number indicates the energy level (The number is called the principal quantum number.). 2. A letter indicates the type of orbital; s, p, d, f.
Worksheet 4 - Electron Configurations. Worksheet 5 - Orbital and Energy Level Diagrams. Worksheet 6 - Quantum Numbers ... electron configurations for each of the following atoms. phosphorus. 1s. 2. 2s. 2. 2p. 6. 3s. 2. 3p. 3 [Ne]3s. 2. 3p. 3. ... Identify and correct the errors in each of the following valence shell orbital diagrams ...
Electron Configurations Worksheet. Write the complete . ground state electron configurations. and . orbital notations. for the following: # of e- Element (atom) e- configuration Orbital Notations/ diagrams
Electron configuration and orbital diagram worksheet pdf The purpose of introducing quantum numbers is to show that the similarity of electron arrays or electron compositions leads to similarities and differences in the characteristics of elements.
•To determine the electron configuration of any of the first 38 elements of the periodic table •To determine the identity of an element from its electron configuration •To complete an orbital diagram using arrows to represent electrons
Electron Configuration Worksheets Awesome Electron Configuration Exercise. An electron diagram represents the location of each electron in an atom's orbit. When a source of light activates an atom, an electric field is formed that imparts energy to the atom and allows it to create a molecule. This process also generates an electron orbital ...
Electron configuration worksheet with answers *free* electron configuration worksheet with answers. Fillable electron configuration and the periodic table. Electron configurations worksheet answers write the complete ground state electron configu rations for the following 1 lithium 1s22s1 2 oxygen electron configuration worksheet pdf.

Solved Quantum Theory Periodlc Treids Workshert Whot Element Represented The A Ectranic Contirtion Ls 25 20835 Jp Wnat Element Represented The Atomic Orbital Diagram Dron He Atcmic Orbital Diagram For Mn Write The Electron Ccnfiguration For
8. The following diagram shows 5 different energy levels (n) that electrons can occupy. Use the diagram to answer each of the following questions: a. EXAMPLE Give a situation that could result in an electron making a quantum leap. An electron gains energy and moves from n=1 to n= 3. b. Give a situation that would result in an electron giving ...
18) Which Groups have a d-orbital as the last orbital? 3 - 12. Which section of the table is left? What is trend of f-orbital elements? Lanthanide and actinide. What is the relationship between the group number and number of valence . electrons? How many valance electrons. For each of the following, give only the last term of the electron ...
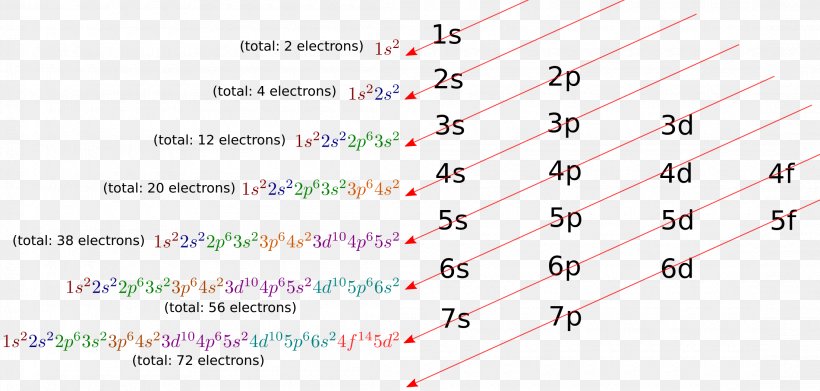
orbitals have one electron. Then additional electrons enter each orbital until 2 . electrons are in each orbital. Once all orbitals in a sublevel are filled (each with 2 . electrons), the next electron enters the next higher energy sublevel. The Aufbau diagram below illustrates the order of filling orbitals and sublevels.

Electron Configuration Worksheet Electron Configuration Acirc Euro Ldquo Ch 4 Chem Use The Patterns Within
Part A – Orbital Diagrams & Longhand Electron Configuration. Use the patterns within the periodic table to draw orbital diagrams and write longhand electron configurations for the following atoms. Symbol # e- Orbital Diagram and. Longhand Electron Configuration Mg P V Ge Part B – Shorthand Electron Configuration
Name _____per_____ Orbital Diagrams and Electron Configuration Worksheet: Part A: Using the Periodic Table, first give the number of protons and electrons in the neutral atom of the given element. On the line above the brackets, write the electron configuration for each element.
Since there is 1 electron in the box labeled 1s, we say the H electron configuration in orbital notation is 1s 1. The orbital notation can also be interpreted as quantum numbers, where the principal quantum number n is the energy level (1 before the s), the azimuthal quantum number corresponds to the letter s, and the spin quantum number is +1/2.
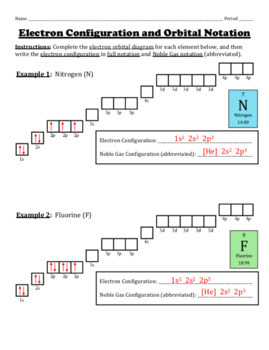
Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1. Nitrogen IS 35 2. Chlorine Is 25 3. Sodium Is 2s 35 I 4. Neon 3p 3p 3p IS 25 . Electron Configuration Practice Chemistry 5. Nickel Name :
Electron configuration and orbital diagram worksheet. Diagram] orbital diagram worksheet key full version hd quality worksheet key. Diagram] orbital diagram worksheet key full version hd quality worksheet key. Electron electrons orbital configuration sequence diagram structure worksheet. Manual for daewoo 1550xl skid steer orbital diagram chem worksheet answers gehl axles as orbital diagram ...
Ground-State Electron Configuration The arrangement of electrons in the atom is called the electron configuration. The aufbau principle states that each electron occupies the lowest energy orbital available. This is an AUFBAU diagram
View Electron Configurations and Orbital Diagrams Worksheet_Smart.pdf from CHM 102 at Northern Virginia Community College. Kavya Smart Calcium Ca Nickel Ni Carbon C Xenon Xe Protactinium Pa Sulfur
10. Which element has the following orbital diagram? 11. Using arrows, show how the following orbitals will fill with electrons. Electron Configuration 1s 2s 2p 3s 3p 4s 3d Mg 1s22s22p63s2 Cl 1s22s22p63s23p5 Si 1s22s22p63s23p2 Ti 1s22s22p63s23p64s23d2 12. Write the complete electron configuration for each atom on the blank line.

Atomic Orbital Electron Configuration Molecular Orbital Diagram Iron Ferric Iron Angle White Electronics Png Pngwing
Symbol # e- Orbital Diagram and Longhand Electron Configuration 1. Mg 1s 22s2 2p6 3s 2. P 1s2 22s2 32p6 3s 3p 3. V 1s2 6 2s2 2p 3s2 3p6 4s23d3 4. Ge 1s 22s 2p63s 3p64s23d104p2 PART B – SHORTHAND ELECTRON CONFIGURATION Use the patterns within the periodic table to write the shorthand electron configurations for the following elements. Symbol # e- Shorthand Electron Configuration 5. Ca [Ar] 4s2 6. Pb [Xe]6s24f145d106p2 7. F [He]2s22p5 8. Pd [Kr]5s24d8
Electron Configuration For Elements Electron Configuration Ch 4 Chem Electron Configurations Studocu
Electron Configuration Chemistry Atomic Orbital Png 2200x1050px Electron Configuration Atom Atomic Orbital Atomic Physics Block Download

Writing Electron Configurations And Drawing Orbital Central School Acirc Euro Cent Ap Chemistry Name Chapter

Electron Configuration Practice Electron Configuration Practice Worksheet On A Separate Piece On Paper Write The
















0 Response to "36 electron configuration and orbital diagram worksheet"
Post a Comment