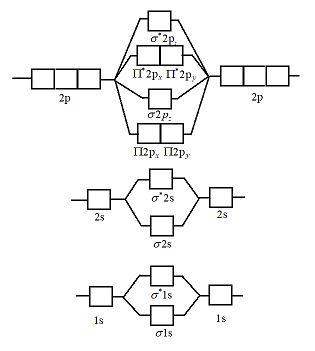
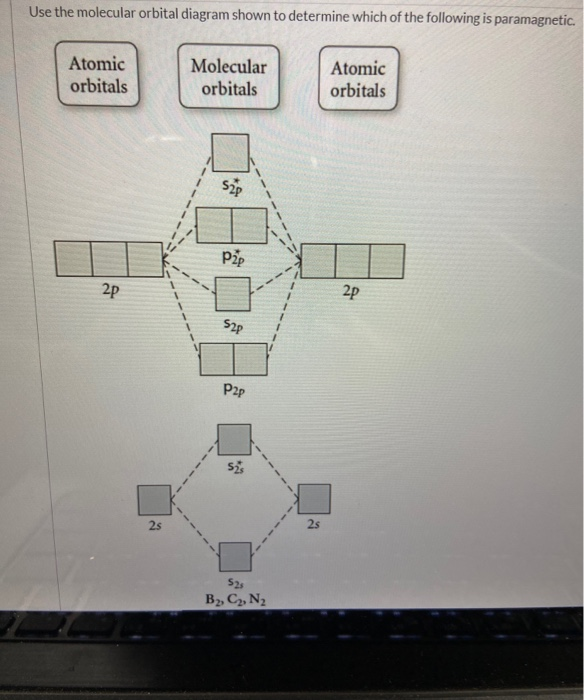
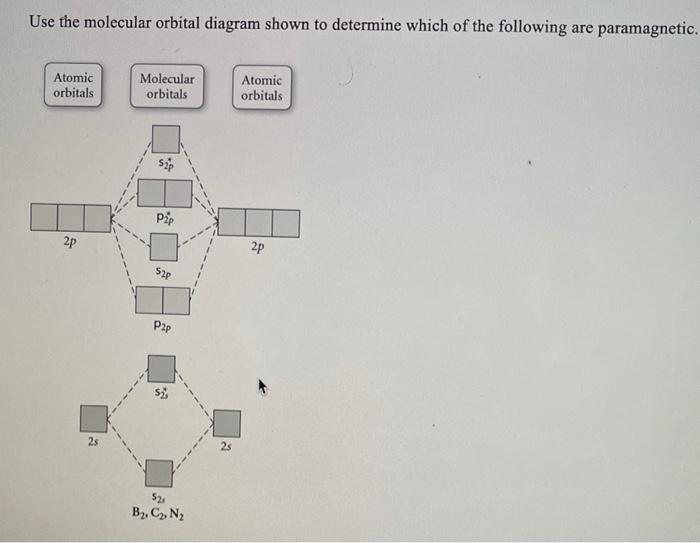
40 Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Are Paramagnetic.
Use The Molecular Orbital Diagram Shown To Determine Which Of... 1 draw the molecular orbital diagrams to determine which of the following is most stable. B f22 c ne22 d o22 e f22 2 use molecular orbital diagrams to determine which of the following are paramagnetic. Solved Draw The Molecular Diagram Shown To Determine Whic. How to identify if a molecule is paramagnetic or diamagnetic - Quora Paramagnetic- if total no. of electrons in the molecule are 10,16 or odd electrons then the molecule is paramagnetic. Paramagnetism is due to the presence of at least one unpaired electron in the molecule. The molecules of simple paramagnetic compounds usually contain odd numbers of...
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Label the molecular orbital shown as σ or π, bonding or antibonding and indicate where the node These candidate molecules are then carefully tested to determine side effects, how effectively they For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the...
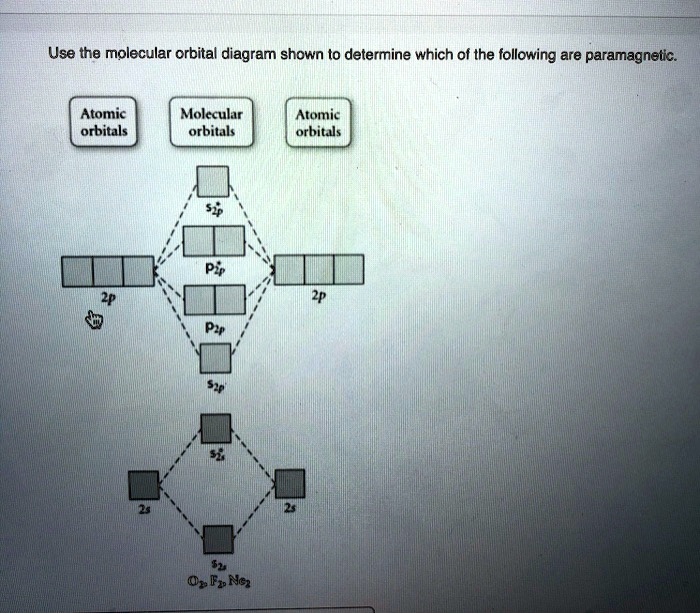
Use the molecular orbital diagram shown to determine which of the following are paramagnetic.
Use the molecular orbital diagram shown to determine which of the Use molecular orbital theory to determine whether f22+ is paramagnetic or diamagnetic. What is the bond order of F22- according to molecular orbital A bonding molecular orbital is of lower energy (more stable) than the atomic orbitals from which it... Which of the following has an incomplete octet... exercises - molecular geometry and bonding theories - chemistry the... 9.10 The diagram that follows shows the highest-energy occupied MOs of a neutral molecule CX, where element X is in the same row of the periodic table as C. (a) Based on the number of electrons, can you determine the identity of X? (b) Would the molecule be diamagnetic or paramagnetic? (c)... OneClass: Use the molecular orbital diagram shown to determine... Related questions. use orbital diagram to determine which of the following are paramagnetic. Which of the following is paramagnetic? Draw the molecular diagram.
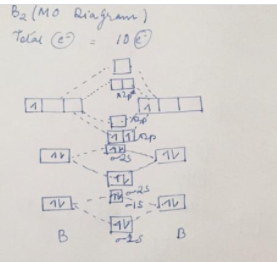
Use the molecular orbital diagram shown to determine which of the following are paramagnetic.. Use the molecular orbital diagram shown to determine which Chemistry helpWhich of the following are true statements about antibonding molecular orbitals? . Select all that apply:a. as For this assignment, you will write atomic diagrams for 10 atoms, and then diagrams for the ions that they might produce, exactly as shown in the examples above. 9.10: Molecular Orbital Theory Predicts that Molecular Oxygen is... The molecular orbital configuration dictates the bond order of the bond. This diagram shows 8 electrons in bonding orbitals and 4 in antibonding orbitals, resulting in a predicted bond Molecular Oxygen is Paramagnetic. We now turn to a molecular orbital description of the bonding in \ceO2. (eg) and molecular geometry(mg) Answer: C 11 52) Use the molecular orbital diagram shown to determine which of the following is most stable. If the molecular geometry causes all of the dipoles to cancel, the molecule will be nonpolar. An example is CF4 where there are four polar bonds, but the dipoles sum to 0 making the... b) By constructing a molecular orbital picture for each of... - Brainly.in ...for each of the following molecules, determine whether it is paramagnetic or diamagnetic. For a compound to be paramagnetic there should be unpaired electron. From the given diagram, we see that in case of Boron, there are two unpaired electron which makes B2 molecule paramagnetic.
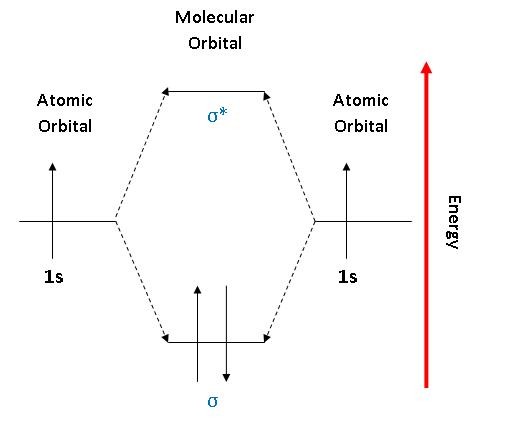
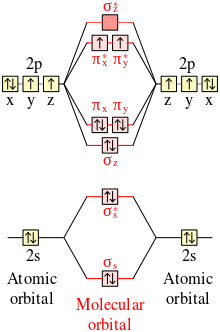
Solved Use the molecular orbital diagram shown to determine ...shown to determine which of the following are paramagnetic Atomic orbitals Molecular You may exercise your right to opt out of the sale of personal information by using this toggle switch. They may be used by those companies to build a profile of your interests and show you relevant... Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. Molecular Orbital Theory Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the geometry of the molecule to which they are applied. Molecular orbitals are obtained by combining the atomic orbitals on the atoms in the molecule. Molecular Orbital Theory | Grandinetti Group Molecular Orbital Theory. The Lewis Structure approach provides an extremely simple method for determining the electronic structure of many To see how we use these concepts in Molecular Orbital Theory, let's start with H2, the simplest of all molecules. The 1s orbitals of the H-atom are...
Which of the following are paramagnetic? - HomeworkLib Use the molecular orbital diagram shown to determine which of the following paramagnetic. Which of the following processes shows a decrease in entropy of the system? Draw the appropriate molecular orbital diagram and determine... Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory. • 269 тыс. просмотров 4 года назад. Thermodynamics, PV Diagrams, Internal Energy, Heat, Work, Isothermal, Adiabatic, Isobaric, Physics. • 687 тыс. просмотров 4 года назад. Using a Micropipette - University of Leicester. Molecular orbital : A molecule in which all the electrons are paired, is... Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12. O2 molecule is paramagnetic because two unpaired electrons are present. One unpaired in π*2py1 and one in π*2pz1. (Get Answer) - Use the molecular orbital diagram shown to... ...orbital diagram shown to determine which of the following is paramagnetic. (a) O^2-_2 (b) Ne^2+_2 (c) O^2-_2 (d) F_2+_2 (e) None of these is paramagnetic. Question 3 of 16 Map& Mapoob sapling leaning Construct the molecular orbital diagram for He and then identify the bond order.
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories The key is to first identify what molecule they are asking you to draw and then determine which of the following categories it belongs to.
How to Tell If an Element Is Paramagnetic or Diamagnetic So paramagnetic materials are also diamagnetic, but because paramagnetism is stronger, that You can determine whether the net effect in a sample is diamagnetic or paramagnetic by Paramagnetic vs Diamagnetic Example. Which of the following elements would be expected to be paramagnetic?
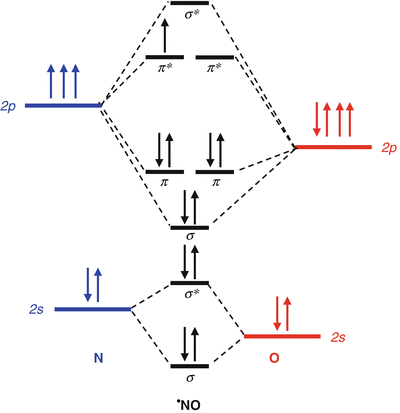
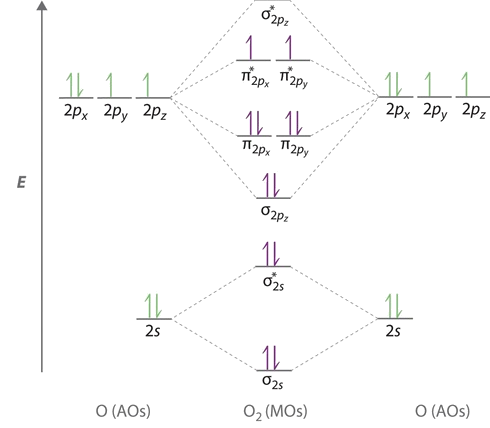
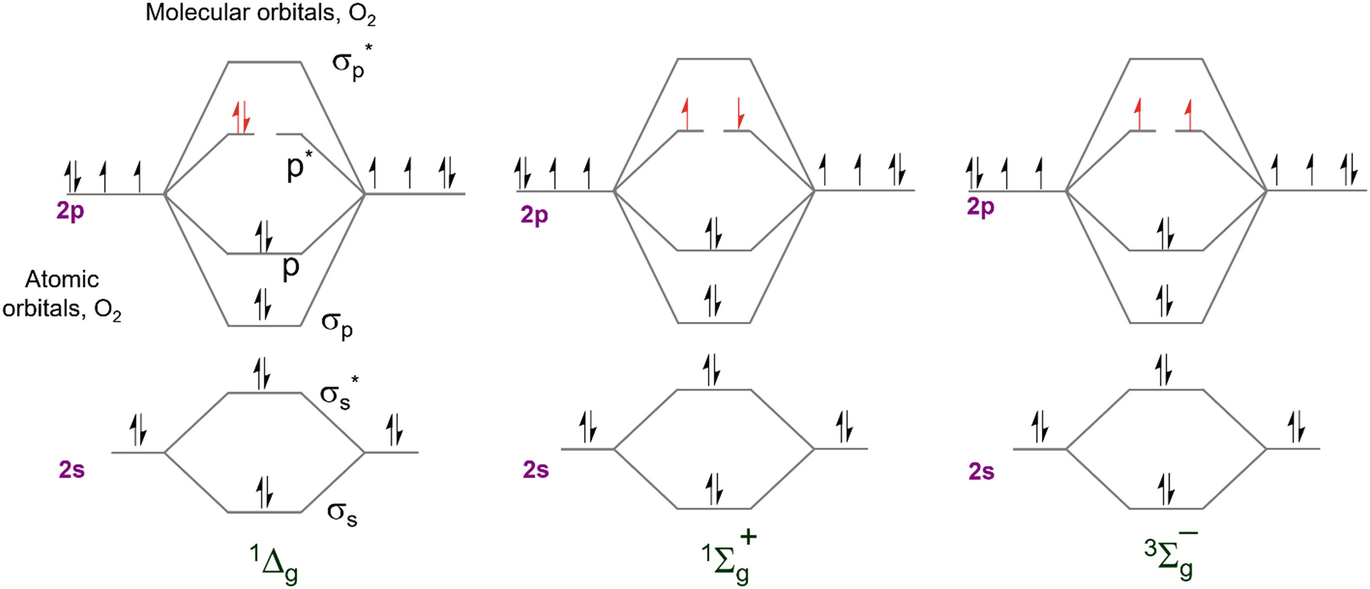
Molecular Orbital Theory: Explanation, Illustrations and... - Embibe Molecular Orbital Theory: It is used to define the bonding in molecules which cannot be explained with the The molecular orbital energy level diagram for dioxygen molecule is shown below However, if electrons singly occupy one or more molecular orbitals, it is said to be paramagnetic.
SOLVED:Use the molecular orbital diagram shown to determine... So here we're just looking at a couple different orbital type. So looking on our s and R P Orbital's and we're gonna be defining all of the shapes. Draw the following orbitals: a. 3s orbital b. 4s orbital c. 3p orbital.
8.4 Molecular Orbital Theory - Chemistry Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. These candidate molecules are then carefully tested to determine side effects, how effectively they can be transported through the body, and other factors.
1 -Use the molecular orbital diagram shown to determine which of... A: Following are the steps to synthesise terpinolene from a straight chain alkanes. Q: Stua, he following sketch of a molecular orbital (MO) in a homonuclear diatomic molecule. This MO wa... A: A molecular orbital is formed by overlapping of atomic orbitals.
PDF Microsoft Word - W-12. Worksheet 2. Molecular Orbital Theory.docx The molecular orbital diagram below may be used for the following problem(s). However, the diagram will still yield the correct bond order and magnetic behavior for these molecules. Use molecular orbital theory to determine if the molecules are. paramagnetic or diamagnetic.
Use The Molecular Orbital Diagram Shown To Determine Which Of... How to draw the c n molecular orbital diagram for the sigma bond in hcn. B f22 c ne22 d o22 e f22 2 use molecular orbital diagrams to determine which of the following are paramagnetic.
chem test 3 (ch 6&7) Flashcards | Quizlet Use the molecular orbital diagram shown to determine which of the following are paramagnetic. Both O2 and O2− are paramagnetic. Formaldehyde, an organic compound frequently used to preserve biological specimens, has the formula H2CO. What is the expected...
PDF Principles of Chemical Science, Solutions for Lecture 13: Molecular... LECTURE 13. 1. Draw a molecular orbital diagram and determine the bond order expected for LECTURE 13. 3. (a) Draw a MO diagram for the valence electrons of BC. Label all atomic and 4. For each of the following molecules, (i) write the valence electron configuration (Your answer should be...
Use the molecular orbital diagram shown to determine | Course Hero 5.E) B22⁺Answer: D Diff: 5 Page Ref: 10.8 87) Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
Molecular Orbital Theory | Chemistry Label the molecular orbital shown as s or π, bonding or antibonding. Indicate where the nuclei and nodes occur. These candidate molecules are then carefully tested to determine side effects, how effectively they Figure 9. This is the molecular orbital diagram for the homonuclear diatomic Be2+...
OneClass: Use the molecular orbital diagram shown to determine... Related questions. use orbital diagram to determine which of the following are paramagnetic. Which of the following is paramagnetic? Draw the molecular diagram.
exercises - molecular geometry and bonding theories - chemistry the... 9.10 The diagram that follows shows the highest-energy occupied MOs of a neutral molecule CX, where element X is in the same row of the periodic table as C. (a) Based on the number of electrons, can you determine the identity of X? (b) Would the molecule be diamagnetic or paramagnetic? (c)...

Use the molecular orbital diagram shown to determine which of the following are paramagnetic.A. Ne22+ B. O22+ C. F22+ D. O22- E. None of the above are paramagnetic.
Use the molecular orbital diagram shown to determine which of the Use molecular orbital theory to determine whether f22+ is paramagnetic or diamagnetic. What is the bond order of F22- according to molecular orbital A bonding molecular orbital is of lower energy (more stable) than the atomic orbitals from which it... Which of the following has an incomplete octet...
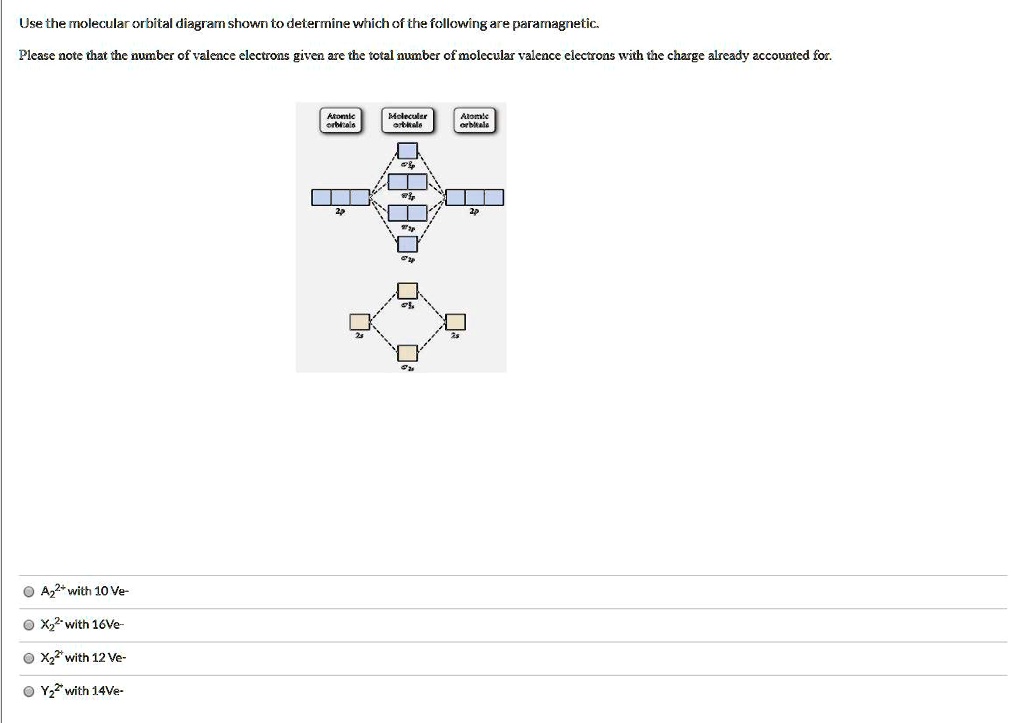

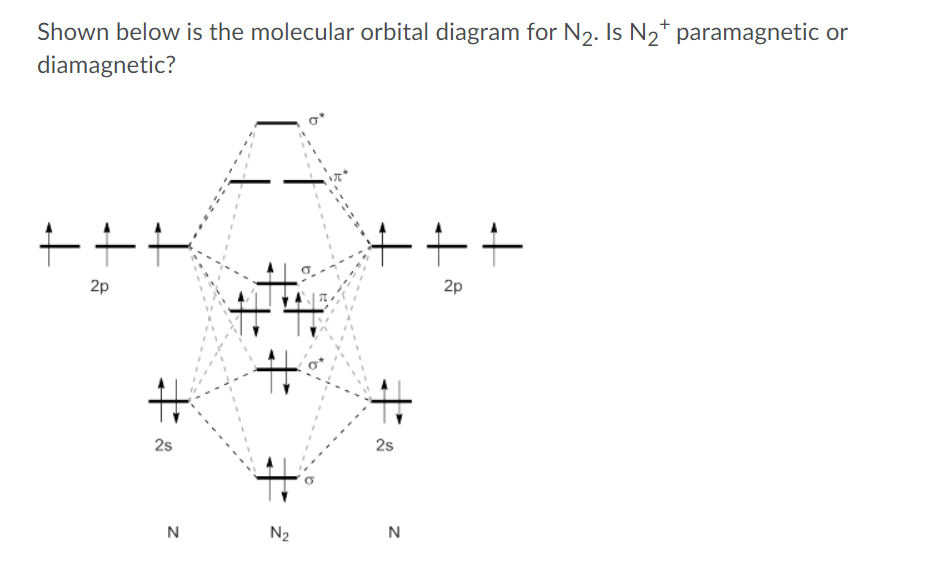


















0 Response to "40 Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Are Paramagnetic."
Post a Comment